推荐产品
等級
certified reference material
品質等級
形狀
liquid
特點
(Snap-N-Spike®)
包裝
ampule of 1 mL
製造商/商標名
Cerilliant®
濃度
1.0 mg/mL in acetonitrile
技術
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
應用
clinical testing
格式
single component solution
運輸包裝
dry ice
儲存溫度
−70°C
InChI
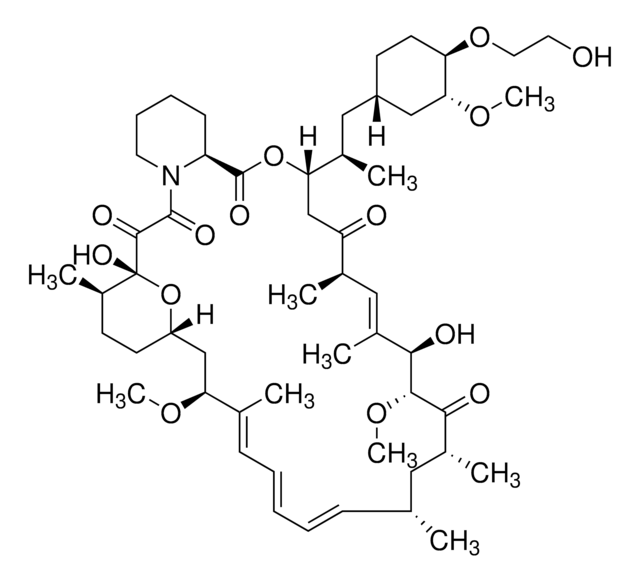
1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1
InChI 密鑰
HKVAMNSJSFKALM-GKUWKFKPSA-N
基因資訊
human ... FKBP1A(2280)
一般說明
應用
- Everolimus as a therapy for hepatoblastoma: Research demonstrates that Everolimus can induce autophagy-dependent ferroptosis in hepatoblastoma cells, highlighting its potential as a therapeutic agent in oncology research. This study provides insight into the mechanisms by which Everolimus can be utilized to target cancer cells through cell death pathways (Huang et al., 2024).
- Understanding oral mucosal injuries from mTOR inhibitors: A new hypothesis posits that oral mucosal injuries associated with mTOR inhibitors like Everolimus result from disruptions in cellular stress and apoptotic pathways. This study underscores the importance of understanding side effects in the context of targeted therapy for conditions such as cancers and immunosuppression (Sonis and Villa, 2023).
- Micellar formulation of Everolimus for neurological disorders: A stable micellar formulation of Everolimus (RAD001) has been developed for intracerebroventricular delivery, aimed at treating Alzheimer′s Disease and other neurological disorders. This formulation allows for direct brain administration, potentially enhancing the drug′s efficacy and safety profile (Gianessi et al., 2023).
- Pharmacokinetics in epilepsy treatment: The population pharmacokinetics of Everolimus were studied in patients with seizures associated with focal cortical dysplasia. This research aids in understanding the drug′s behavior in a specific neurological context, providing a foundation for dosing adjustments and therapeutic monitoring (Park et al., 2023).
法律資訊
相關產品
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
35.6 °F - closed cup
閃點(°C)
2.0 °C - closed cup
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门