推荐产品
形狀
powder
包裝
pkg of 1 × 1 mg (860464P-1mg)
製造商/商標名
Avanti Polar Lipids 860464P
脂質類型
sphingolipids
bioactive lipids
運輸包裝
dry ice
儲存溫度
−20°C
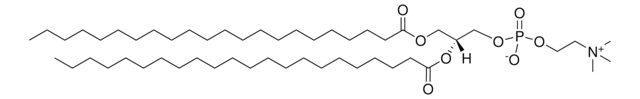
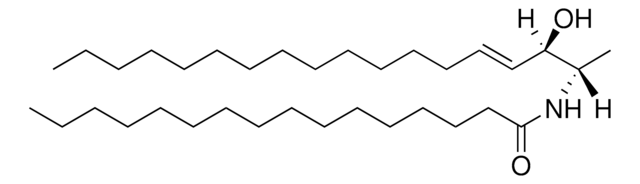
SMILES 字串
C[C@]([H])(NC(CCCCCCCCCCCCC/C=C\CCCCCCCC)=O)[C@]([H])(O)CCCCCCCCCCCCCCC
一般說明
1-deoxysphinganine is an atypical sphingoid base, which lacks a 1-hydroxyl group.
應用
N-C24:1-deoxysphinganine or N-nervonoyl-1-deoxysphinganine (m18:0/24:1) has been used as a standard in the quantitation of atypical sphingoid bases in biological samples by reverse-phase liquid chromatography coupled to electrospray ionization tandem mass spectrometry (LC-MS/MS).
生化/生理作用
1-deoxysphinganine might be toxic to cancer cells. It is a potential biomarker for type 2 diabetes. 1-deoxysphinganine acts as a cytotoxic lipid for insulin producing cells.
包裝
5 mL Amber Glass Screw Cap Vial (860464P-1mg)
法律資訊
Avanti Research is a trademark of Avanti Polar Lipids, LLC
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
Noemi Jiménez-Rojo et al.
Biophysical journal, 107(12), 2850-2859 (2014-12-18)
Ceramides and dihydroceramides are N-acyl derivatives of sphingosine and sphinganine, respectively, which are the major sphingoid-base backbones of mammals. Recent studies have found that mammals, like certain other organisms, also produce 1-deoxy-(dihydro)ceramides (1-deoxyDHCers) that contain sphingoid bases lacking the 1-hydroxyl-
Junliang Wan et al.
Journal of agricultural and food chemistry, 67(46), 12953-12961 (2019-10-23)
Most common sphingolipids are comprised of "typical" sphingoid bases (sphinganine, sphingosine, and structurally related compounds) and are produced via the condensation of l-serine with a fatty acyl-CoA by serine palmitoyltransferase. Some organisms, including mammals, also produce "atypical" sphingoid bases that
Sarah T Pruett et al.
Journal of lipid research, 49(8), 1621-1639 (2008-05-24)
"Sphingosin" was first described by J. L. W. Thudichum in 1884 and structurally characterized as 2S,3R,4E-2-aminooctadec-4-ene-1,3-diol in 1947 by Herb Carter, who also proposed the designation of "lipides derived from sphingosine as sphingolipides." This category of amino alcohols is now
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![16:0-d31-18:1 PG 1-palmitoyl-d31-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (sodium salt), chloroform](/deepweb/assets/sigmaaldrich/product/structures/392/823/d104ed37-ed4a-4f66-8294-a20d5f9085b4/640/d104ed37-ed4a-4f66-8294-a20d5f9085b4.png)