推荐产品
生物源
synthetic
品質等級
等級
FG
Fragrance grade
Halal
Kosher
agency
follows IFRA guidelines
meets purity specifications of JECFA
法律遵循
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
化驗
≥99%
顏色
light yellow to orange, darkens over time
折射率
n20/D 1.5070 (lit.)
bp
67 °C/10 mmHg (lit.)
mp
26-28 °C (lit.)
密度
1.098 g/mL at 25 °C (lit.)
應用
flavors and fragrances
文件
see Safety & Documentation for available documents
食物過敏原
no known allergens
香料過敏原
no known allergens
感官的
almond; caramel; cocoa; nutty; brown
儲存溫度
2-8°C
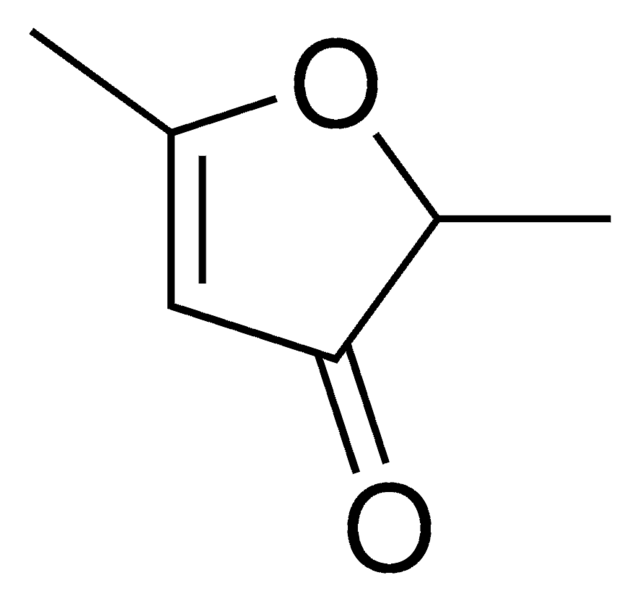
SMILES 字串
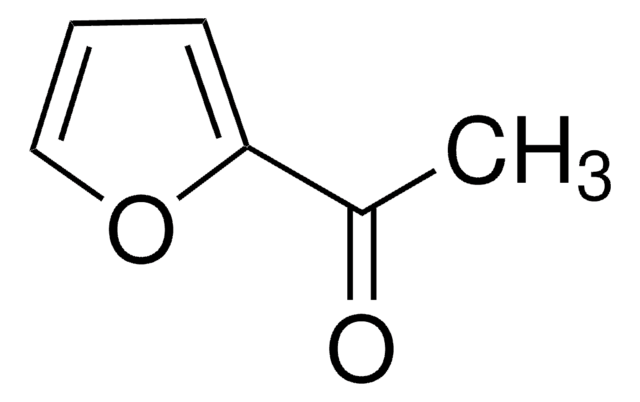
CC(=O)c1ccco1
InChI
1S/C6H6O2/c1-5(7)6-3-2-4-8-6/h2-4H,1H3
InChI 密鑰
IEMMBWWQXVXBEU-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
生化/生理作用
其他說明
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 2 Inhalation - Acute Tox. 3 Oral
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
159.8 °F - closed cup
閃點(°C)
71 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
实验方案
Separation of 5-Hydroxymethyl-2-furaldehyde; Furfuryl alcohol; Furfural; 2-Furyl methyl ketone; 5-Methyl-2-furaldehyde
HPLC Analysis of Furans on Ascentis® Express C18
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门