所有图片(1)
About This Item
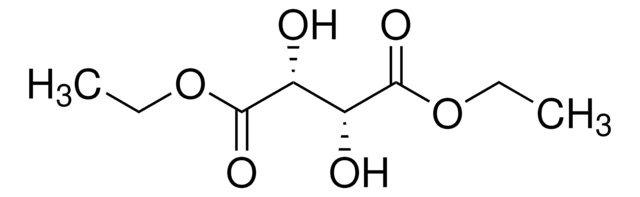
线性分子式:
[-CH(OH)CO2C2H5]2
CAS号:
分子量:
206.19
FEMA號碼:
2378
Beilstein:
1727145
EC號碼:
歐洲委員會號碼:
440
MDL號碼:
分類程式碼代碼:
12164502
PubChem物質ID:
Flavis號碼:
9.446
NACRES:
NA.21
agency:
follows IFRA guidelines
meets purity specifications of JECFA
meets purity specifications of JECFA
推荐产品
生物源
synthetic
品質等級
等級
FG
Fragrance grade
Kosher
agency
follows IFRA guidelines
meets purity specifications of JECFA
法律遵循
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
化驗
≥99%
光學活性
[α]20/D +8.5°, neat
折射率
n20/D 1.446 (lit.)
bp
280 °C (lit.)
密度
1.204 g/mL at 25 °C (lit.)
應用
flavors and fragrances
文件
see Safety & Documentation for available documents
食物過敏原
no known allergens
香料過敏原
no known allergens
感官的
fruity; wine-like
SMILES 字串
CCOC(=O)[C@H](O)[C@@H](O)C(=O)OCC
InChI
1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m1/s1
InChI 密鑰
YSAVZVORKRDODB-PHDIDXHHSA-N
正在寻找类似产品? 访问 产品对比指南
應用
- Synthesis of l-threitol-based crown ethers and their application as enantioselective phase transfer catalyst in Michael additions.: This study synthesizes l-threitol-based crown ethers using diethyl ʟ-tartrate and explores their efficacy as enantioselective phase transfer catalysts in Michael additions, highlighting their potential in asymmetric synthesis (Rapi et al., 2017).
- A facile approach for the synthesis of C13-C24 fragments of maltepolides A, C and D.: This research demonstrates a novel synthesis method for C13-C24 fragments of maltepolides A, C, and D using diethyl ʟ-tartrate, facilitating the study and development of these bioactive compounds (Rao & Srihari, 2016).
- Development of diacyltetrol lipids as activators for the C1 domain of protein kinase C.: This research introduces diacyltetrol lipids synthesized from diethyl ʟ-tartrate, which act as activators for the C1 domain of protein kinase C, offering insights into signal transduction and therapeutic applications (Mamidi et al., 2012).
- Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol.: The paper presents the total synthesis of broussonetine F, utilizing diethyl ʟ-tartrate in an orthoamide Overman rearrangement, showcasing a novel synthetic route for complex natural products (Hama et al., 2011).
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
199.4 °F - closed cup
閃點(°C)
93 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Aman Ullah et al.
Biomacromolecules, 12(10), 3826-3832 (2011-09-06)
Poultry feather quills have been extruded in a twin screw extruder with sodium sulfite treatment as a reducing agent. The effect of four different plasticizers (ethylene glycol, propylene glycol, glycerol, and diethyl tartrate) on the thermoplastic properties was then investigated.
Total synthesis of the light-harvesting carotenoid peridinin.
Thomas Olpp et al.
Angewandte Chemie (International ed. in English), 45(24), 4023-4027 (2006-05-10)
Jiang Weng et al.
The Journal of organic chemistry, 75(9), 3125-3128 (2010-04-17)
A short and practical synthesis of oseltamivir was accomplished in 11 steps from inexpensive and abundant diethyl D-tartrate starting material. This azide-free route featured an asymmetric aza-Henry reaction and a domino nitro-Michael/Horner-Wadsworth-Emmons (HWE) reaction as the key steps to construct
Naoto Hama et al.
Organic letters, 13(4), 616-619 (2011-01-05)
A first total synthesis of broussonetine F from diethyl L-tartrate was achieved. The cornerstone of our synthesis was an orthoamide Overman rearrangement, which provided an allylic amino alcohol with complete diastereoselectivity.
Two-chiral component microemulsion EKC - chiral surfactant and chiral oil. Part 2: diethyl tartrate.
Kimberly A Kahle et al.
Electrophoresis, 28(15), 2644-2657 (2007-06-29)
In this second study on dual-chirality microemulsions containing a chiral surfactant and a chiral oil, a less hydrophobic and lower interfacial tension chiral oil, diethyl tartrate, is employed (Part 1, Foley, J. P. et al.., Electrophoresis, DOI: 10.1002/elps.200600551). Six stereochemical
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门