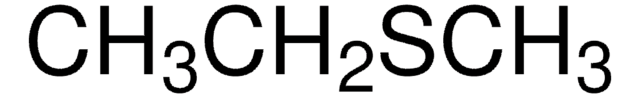推荐产品
蒸汽壓力
18 mmHg ( 25 °C)
品質等級
化驗
99%
折射率
n20/D 1.504 (lit.)
bp
119 °C (lit.)
mp
−96 °C (lit.)
密度
1 g/mL at 25 °C (lit.)
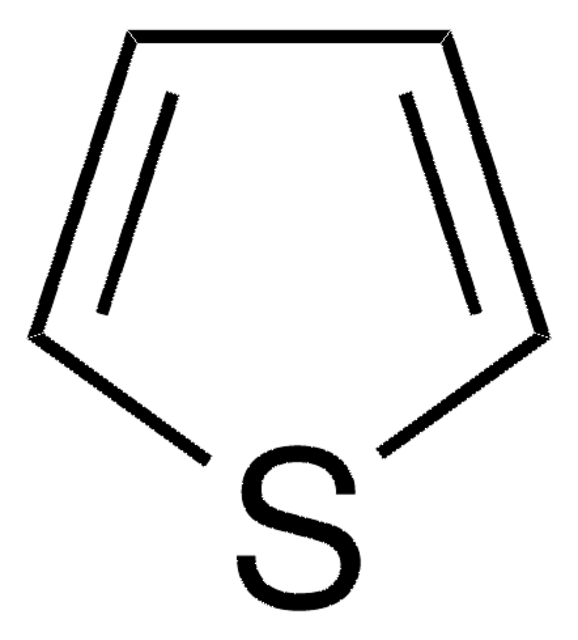
SMILES 字串
C1CCSC1
InChI
1S/C4H8S/c1-2-4-5-3-1/h1-4H2
InChI 密鑰
RAOIDOHSFRTOEL-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
四氢噻吩可用作合成各种环氧化物及其衍生物的试剂。它可用作合成苯并[n.1.0]双环烷烃的催化剂。
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
55.4 °F - closed cup
閃點(°C)
13 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
A new protocol for the in situ generation of aromatic, heteroaromatic, and unsaturated diazo compounds and its application in catalytic and asymmetric epoxidation of carbonyl compounds. Extensive studies to map out scope and limitations, and rationalization of diastereo-and enantioselectivities.
Aggarwal VK, et al.
Journal of the American Chemical Society, 125(36), 10926-10940 (2003)
Tetrahydrothiophene-catalyzed synthesis of benzo [n.1.0] bicycloalkanes.
Ye LW, et al.
The Journal of Organic Chemistry, 72(4), 1335-1340 (2007)
Catalytic asymmetric synthesis of epoxides from aldehydes using sulfur ylides with in situ generation of diazocompounds.
Aggarwal VK, et al.
Angewandte Chemie (International Edition in English), 40(8), 1430-1433 (2001)
Benoit Gautier et al.
Chemistry & biology, 18(12), 1631-1639 (2011-12-27)
Protein-protein interactions play a central role in medicine, and their modulation with small organic compounds remains an enormous challenge. Because it has been noted that the macromolecular complexes modulated to date have a relatively pronounced binding cavity at the interface
Action of three ectopeptidases on corticotropin-releasing factor: metabolism and functional aspects.
James C Ritchie et al.
Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology, 28(1), 22-33 (2002-12-24)
Using purified enzyme preparations, we investigated the actions of angiotensin-converting enzyme, aminopeptidase N, and endopeptidase 24.11 on corticotropin-releasing factor (CRF). The effects of inhibition of these enzymes on CRF action in rat anterior pituitary cultures were also determined. Finally, specific
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持