推荐产品
品質等級
化驗
98%
mp
202-206 °C (lit.)
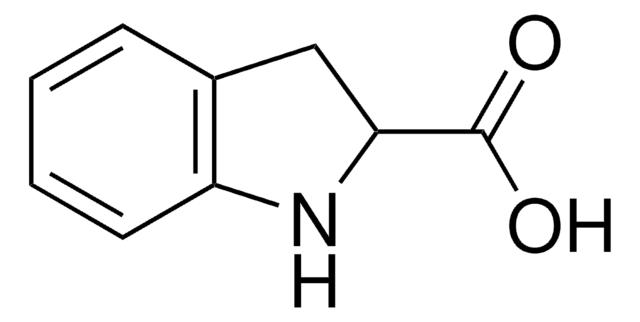
SMILES 字串
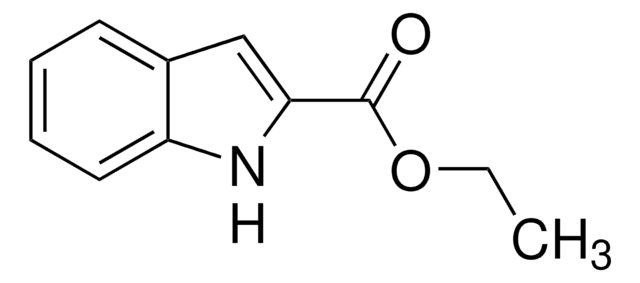
OC(=O)c1cc2ccccc2[nH]1
InChI
1S/C9H7NO2/c11-9(12)8-5-6-3-1-2-4-7(6)10-8/h1-5,10H,(H,11,12)
InChI 密鑰
HCUARRIEZVDMPT-UHFFFAOYSA-N
基因資訊
human ... SRD5A1(6715)
rat ... Grin2a(24409)
正在寻找类似产品? 访问 产品对比指南
應用
- (±)-二溴谷胱甘肽和类似物全合成的反应物
- 吡咯里西啶生物碱(±)-颈花脒合成的反应物
- 肾素霉素G类似物立体选择性制备的反应物
- 通过还原吲哚-2-羧酸以及随后的氧化、缩合、还原、酰胺化和Kharasch自由基环化进行螺氧吲哚吡咯烷制备的反应物
- Pd催化的环化反应反应物
- N,N′-(戊烷)二基双[吲哚甲酰胺]和N,N′-[亚苯基双(亚甲基)]双[吲哚甲酰胺]衍生物制备的反应物
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
James F Dropinski et al.
Bioorganic & medicinal chemistry letters, 15(22), 5035-5038 (2005-09-13)
A series of novel aryl indole-2-carboxylic acids has been identified as potent selective PPARgamma modulators. Their chemical synthesis and in vitro activities are discussed. Compound 5 was selected for in vivo testing in the db/db mouse model of type 2
R Rama Suresh et al.
The Journal of organic chemistry, 77(16), 6959-6969 (2012-07-26)
Two methodologies, one involving Ar-I reactivity and the other through C-H functionalization, for the formation of indolo[2,3-c]pyrane-1-ones via the corresponding allenes, are presented. A highly efficient approach to indolo[2,3-c]pyrane-1-one derivatives through the Pd-catalyzed regioselective annulation of allenes with 3-iodo-1-alkylindole-2-carboxylic acids
Hideyuki Shiozawa et al.
Journal of the American Chemical Society, 124(15), 3914-3919 (2002-04-11)
Glycopeptide antibiotics of the vancomycin group bind to bacterial cell wall analogue precursors, and typically also form dimers. We have studied the interplay between these two sets of noncovalent bonds formed at separate interfaces. Indole-2-carboxylic acid (L) forms a set
Gopinadhan N Anilkumar et al.
Bioorganic & medicinal chemistry letters, 21(18), 5336-5341 (2011-08-16)
SAR development of indole-based palm site inhibitors of HCV NS5B polymerase exemplified by initial indole lead 1 (NS5B IC(50)=0.9 μM, replicon EC(50)>100 μM) is described. Structure-based drug design led to the incorporation of novel heterocyclic moieties at the indole C3-position
R Di Fabio et al.
Journal of medicinal chemistry, 42(18), 3486-3493 (1999-09-10)
A series of analogues of the indole-2-carboxylate GV150526, currently in clinical trials as a potential neuroprotective agent for the control of the cerebral damage after stroke onset, was designed based on previous studies dealing with the electronic features of the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门