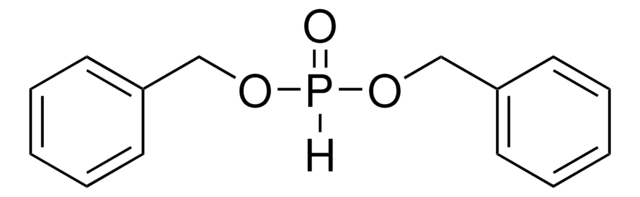
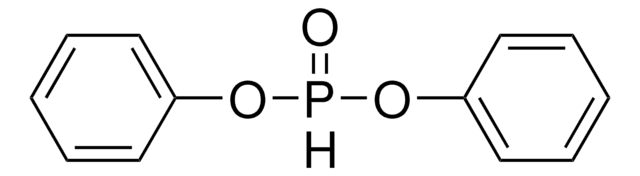
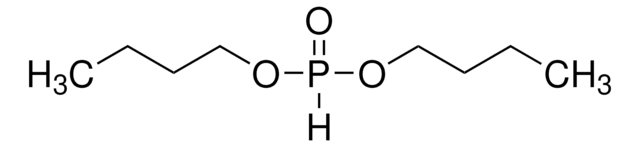
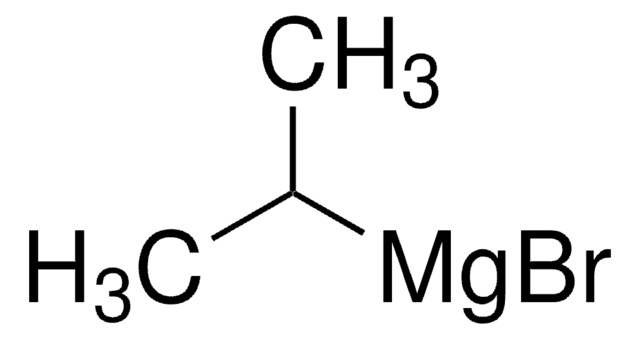
推荐产品
品質等級
化驗
98%
形狀
liquid
折射率
n20/D 1.407 (lit.)
bp
50-51 °C/2 mmHg (lit.)
密度
1.072 g/mL at 25 °C (lit.)
SMILES 字串
[H]P(=O)(OCC)OCC
InChI
1S/C4H11O3P/c1-3-6-8(5)7-4-2/h8H,3-4H2,1-2H3
InChI 密鑰
MJUJXFBTEFXVKU-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Diethyl phosphite is the organophosphorus compound used as a phosphorylating agent in organic synthesis.
應用
Applications of diethyl phosphite (DEP):
- It can undergo condensation with aldehydes or ketones and an amine to form α-aminophosphonates under solvent-free and catalyst-free conditions.
- Reduction of gem-dibromo derivatives using diethyl phosphite in the presence of triethylamine gives monobromocyclopropanes.
- Along with 4-dimethylaminopyridine, it can be used as a ligand for nickel-catalyzed cross-coupling of aryl and heteroaryl bromides, chlorides or sulfonates with arylzinc reagents.
- It can be used for the catalytic asymmetric hydrophosphonylation of enones in the presence of a dinuclear zinc complex to form γ-oxo-phosphonates.
- DEP can undergo Michael addition to α,β-unsaturated malonates to form β-phosphonomalonates in the presence of recyclable nano γ-ferric oxide-pyridine based catalyst.
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Sens. 1B
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
179.6 °F - closed cup
閃點(°C)
82 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Highly Enantioselective 1,4?Addition of Diethyl Phosphite to Enones Using a Dinuclear Zn Catalyst.
Zhao D, et al.
Chemistry?A European Journal, 15(12), 2738-2741 (2009)
A simple and green procedure for the synthesis of α-aminophosphonate by a one-pot three-component condensation of carbonyl compound, amine and diethyl phosphite without solvent and catalyst.
Ranu B C and Hajra A
Green Chemistry, 4(6), 551-554 (2002)
Phospha-michael addition of diethyl phosphite to α,β-unsaturated malonates catalyzed by nano γ-Fe2O3-pyridine based catalyst as a new magnetically recyclable heterogeneous organic base.
Sobhani S, et al.
Applied Catalysis A: General, 454, 145-151 (2013)
Reduction of gem-dibromides with diethyl phosphite.
Hirao T, et al.
The Journal of Organic Chemistry, 46(18), 3745-3747 (1981)
An efficient Negishi cross-coupling reaction catalyzed by nickel (II) and diethyl phosphite.
Gavryushin A, et al.
Tetrahedron, 62(32), 7521-7533 (2006)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门