推荐产品
化驗
99%
形狀
powder
bp
297-298 °C (lit.)
mp
65-67 °C (lit.)
SMILES 字串
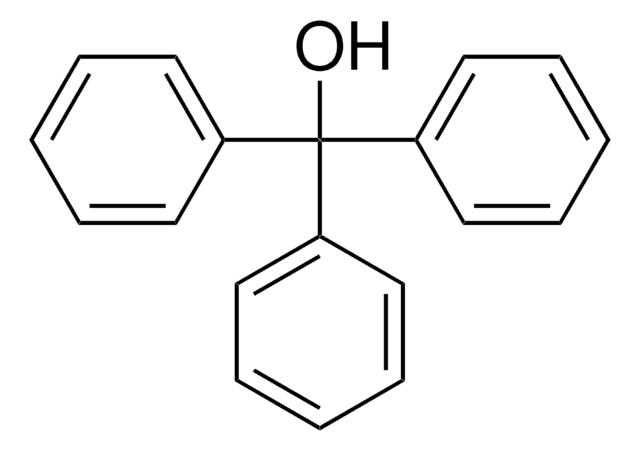
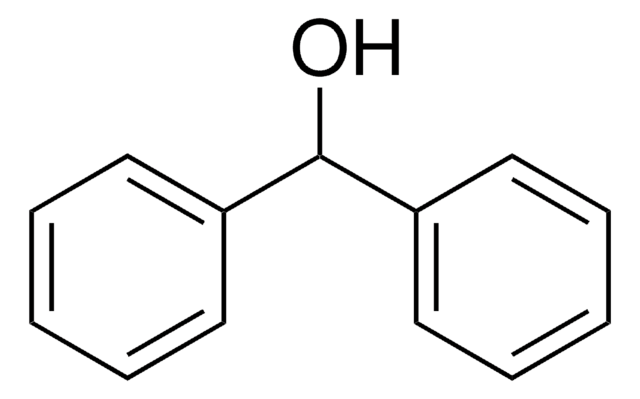
OC(c1ccccc1)c2ccccc2
InChI
1S/C13H12O/c14-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,13-14H
InChI 密鑰
QILSFLSDHQAZET-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
二苯基甲醇用于卡宾催化的α,α-二取代羧酸酯的动态动力学拆分。也可用于游离碱和 Ni (II) 卟啉配合物的手性拆分。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Helimeric porphyrinoids: stereostructure and chiral resolution of meso-tetraarylmorpholinochlorins.
Bru?ckner C, et al.
Journal of the American Chemical Society, 133(22), 8740-8752 (2011)
Masanori Ichikawa et al.
Bioorganic & medicinal chemistry, 20(9), 3072-3093 (2012-04-03)
In the present article, we have reported the design, synthesis, and identification of highly potent benzhydrol derivatives as squalene synthase inhibitors (compound 1). Unfortunately, the in vivo efficacies of the compounds were not enough for acquiring the clinical candidate. We
Xiao-Dong Ma et al.
Bioorganic & medicinal chemistry, 19(16), 4704-4709 (2011-07-27)
A series of (±)-benzhydrol derivatives featuring the essential sulfonamide group at the para position on the C-ring were synthesized and evaluated for the potential anti-HIV activity in C8166 cells. Most of these analogues demonstrated low concentration inhibitory activity with EC(50)
Carbene-Catalyzed Dynamic Kinetic Resolution of Carboxylic Esters.
Chen X, et al.
Journal of the American Chemical Society, 138(23), 7212-7215 (2016)
Alkylation of alcohols for gas chromatographic analysis by a phase transfer catalysis technique. Evaluation of benzhydrol benzylation in a one-phase system.
H Brink et al.
Acta pharmaceutica Suecica, 16(4), 247-262 (1979-01-01)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门