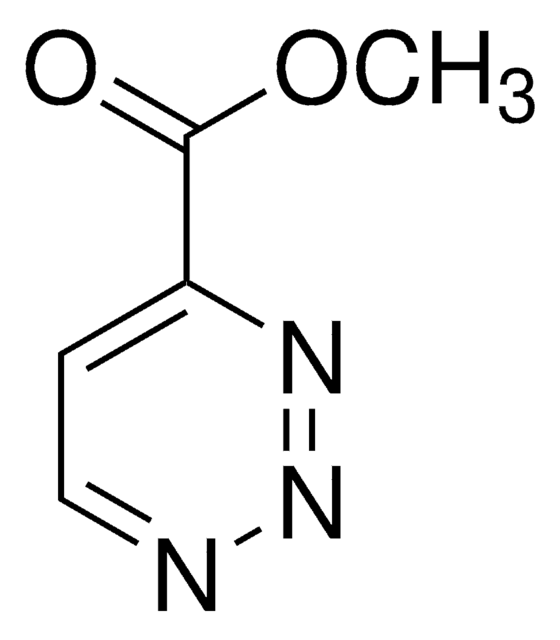
推荐产品
化驗
≥95%
形狀
solid
儲存溫度
2-8°C
SMILES 字串
O=C(OCC)C1=NN=NC(C(OCC)=O)=C1
InChI
1S/C9H11N3O4/c1-3-15-8(13)6-5-7(11-12-10-6)9(14)16-4-2/h5H,3-4H2,1-2H3
InChI 密鑰
WSXMBZCJYIARRQ-UHFFFAOYSA-N
應用
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
商品
The inverse electron demand Diels-Alder reactions of electron-deficient heterocycles are significant cycloaddition reactions for the total synthesis of natural products containing highly substituted and functionalized heteroaromatic ring systems.
The inverse electron demand Diels-Alder reactions of electron-deficient heterocycles are significant cycloaddition reactions for the total synthesis of natural products containing highly substituted and functionalized heteroaromatic ring systems.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门