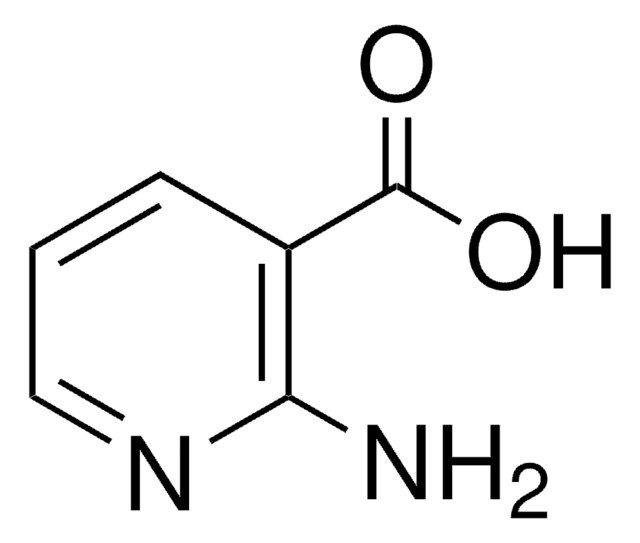
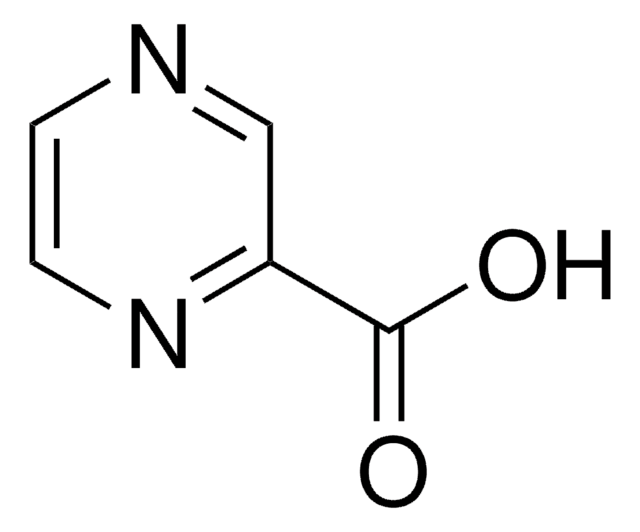
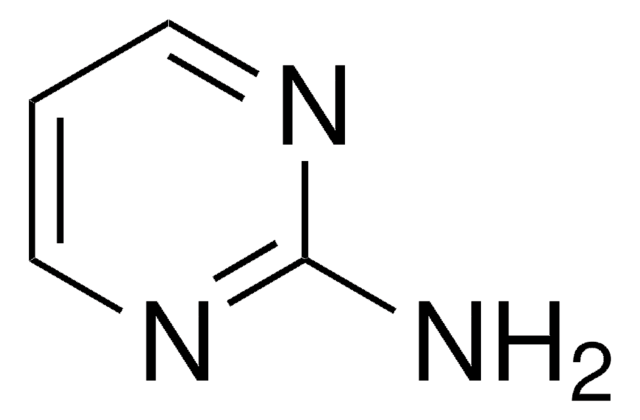
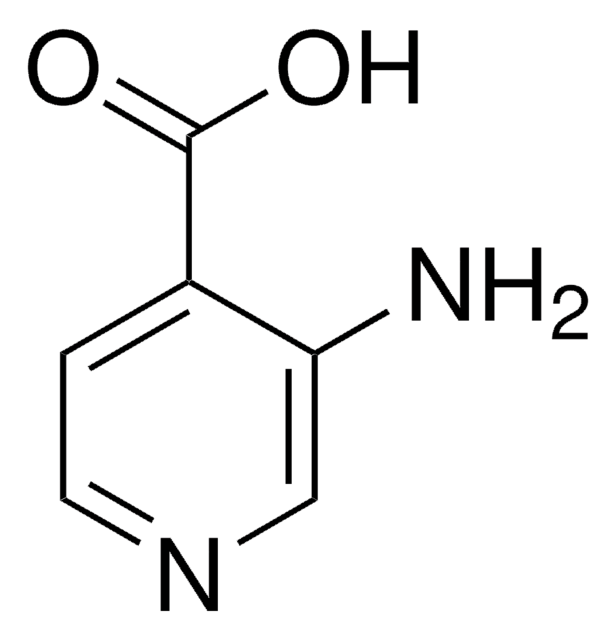
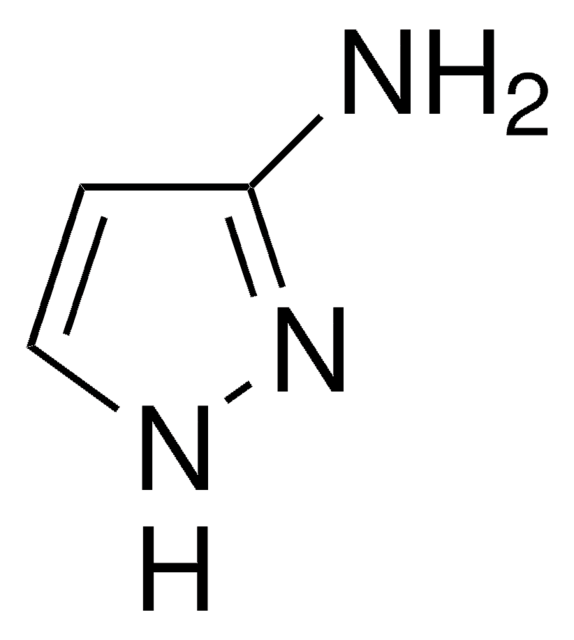
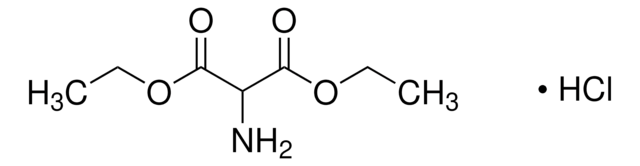
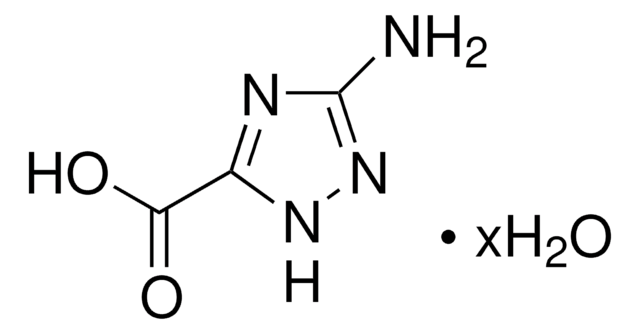
推荐产品
化驗
≥99%
形狀
powder
mp
205-210 °C (dec.) (lit.)
SMILES 字串
Nc1nccnc1C(O)=O
InChI
1S/C5H5N3O2/c6-4-3(5(9)10)7-1-2-8-4/h1-2H,(H2,6,8)(H,9,10)
InChI 密鑰
ZAGZIOYVEIDDJA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
A J Dobson et al.
Acta crystallographica. Section C, Crystal structure communications, 52 ( Pt 6), 1512-1514 (1996-06-15)
3-Aminopyrazine-2-carboxylic acid, C5H5N3O2, displays an extensive network of intra- and intermolecular hydrogen bonds which are undoubtedly responsible for the modest values of the displacement parameters. H-atom transfer to the ring N atoms did not occur and the carboxy and amino
Ghada Bouz et al.
Molecules (Basel, Switzerland), 24(7) (2019-03-31)
We report the design, synthesis, and in vitro antimicrobial activity of a series of N-substituted 3-aminopyrazine-2-carboxamides with free amino groups in position 3 on the pyrazine ring. Based on various substituents on the carboxamidic moiety, the series is subdivided into
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门