推荐产品
化驗
95%
形狀
powder or crystals
反應適用性
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
reaction type: Cross Couplings
reagent type: ligand
mp
164-166 °C (lit.)
165.5 °C
官能基
phosphine
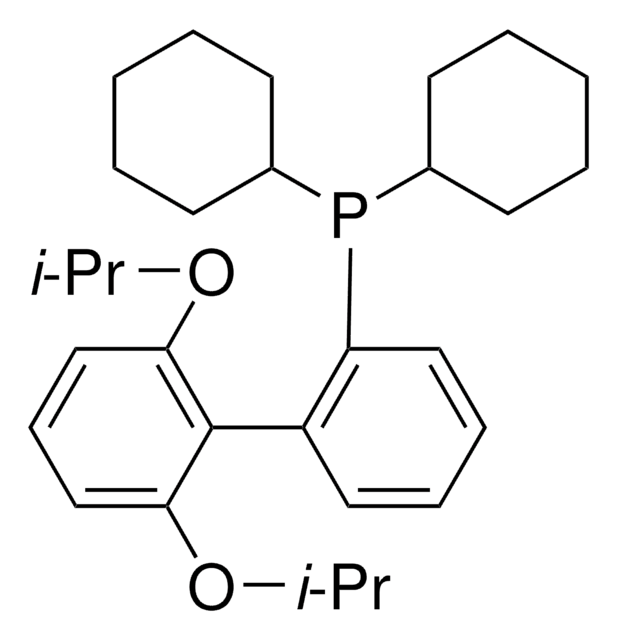
SMILES 字串
COc1cccc(OC)c1-c2ccccc2P(C3CCCCC3)C4CCCCC4
InChI
1S/C26H35O2P/c1-27-23-17-11-18-24(28-2)26(23)22-16-9-10-19-25(22)29(20-12-5-3-6-13-20)21-14-7-4-8-15-21/h9-11,16-21H,3-8,12-15H2,1-2H3
InChI 密鑰
VNFWTIYUKDMAOP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
SPhos [2-Dicyclohexylphosphino-2′, 6′-dimethoxybiphenyl] is an air-stable, electron-rich biaryl phosphine ligand developed by the Buchwald group to enhance the reactivity of palladium catalysis during cross-coupling reactions.
應用
SPhos may be used as a ligand in the following processes:
- Palladium catalyzed Suzuki-Miyaura cross-coupling reaction between Boc-protected aminomethyltrifluoroborate and aryl chlorides or hetaryl chlorides to form the corresponding aminomethylarenes.
- Palladium catalyzed Suzuki-Miyaura cross-coupling reaction between 4-methyl-substituted piperidinylzinc reagent and different aryl or heteroaryl iodides to form various substituted piperidines.
- Intramolecular Suzuki-Miyaura coupling to form the 18-membered macrocyclic ring during the multi-step synthesis of riccardin C.
Utilized in conjunction with palladium to form a highly active catalyst for C-N bond formation
用于Suzuki-Miyaura偶联反应的高度通用配体; 芳基氯、受阻联芳基、杂联芳基。
法律資訊
使用涉及的专利号:US 6307087;EP 1097158;JP 5758844;CA 2336691
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Synthesis of riccardin C and its seven analogues. Part 1: The role of their phenolic hydroxy groups as LXRa agonists.
Hioki H, et al.
Bioorganic & Medicinal Chemistry Letters, 19(3), 738-741 (2009)
Seel S, et al.
Journal of the American Chemical Society, 133(13), 4774-4777 (2011)
Sustainable Fe?ppm Pd nanoparticle catalysis of Suzuki-Miyaura cross-couplings in water
Handa, Sachin, et al.
Science, 349.6252, 1087-1091 (2015)
Pd-catalyzed Suzuki?Miyaura reactions of aryl halides using bulky biarylmonophosphine ligands
Altman, Ryan A., and Stephen L. Buchwald
Nature Protocols, 2.12 (2007)
A general solution for unstable boronic acids: slow-release cross-coupling from air-stable MIDA boronates
Knapp, David M., Eric P. Gillis, and Martin D. Burke
Journal of the American Chemical Society, 131.20, 6961-6963 (2009)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门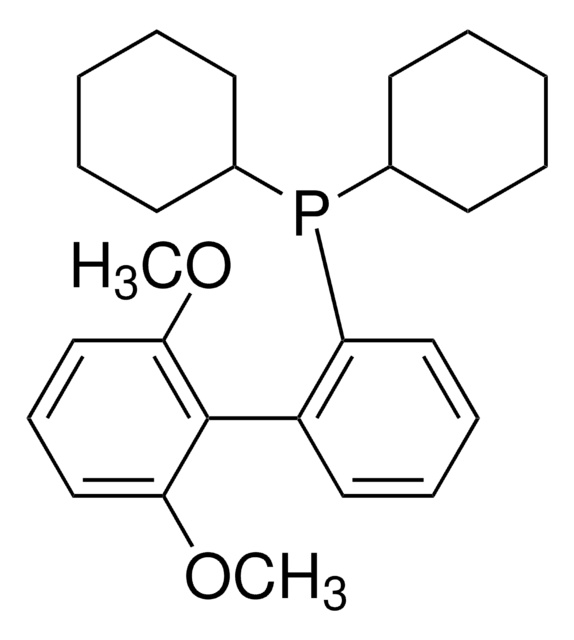


![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)