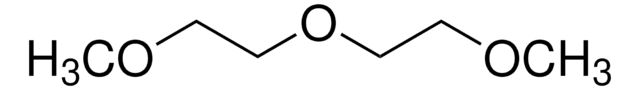
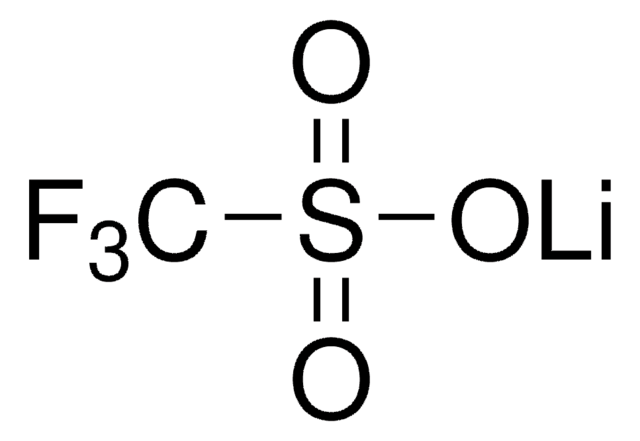
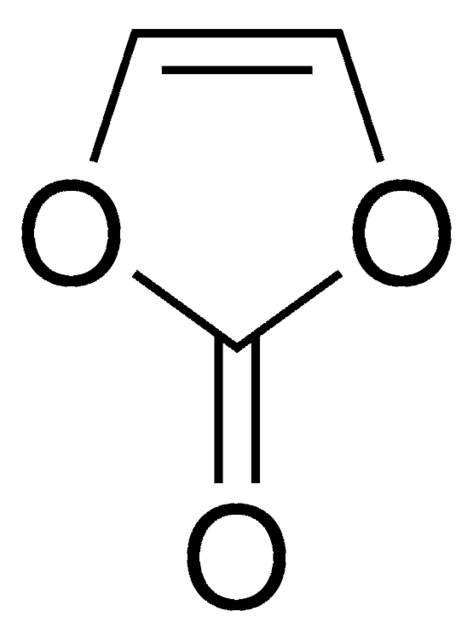
推荐产品
等級
anhydrous
化驗
≥98%
形狀
powder
mp
293-300 °C (dec.) (lit.)
應用
battery manufacturing
SMILES 字串
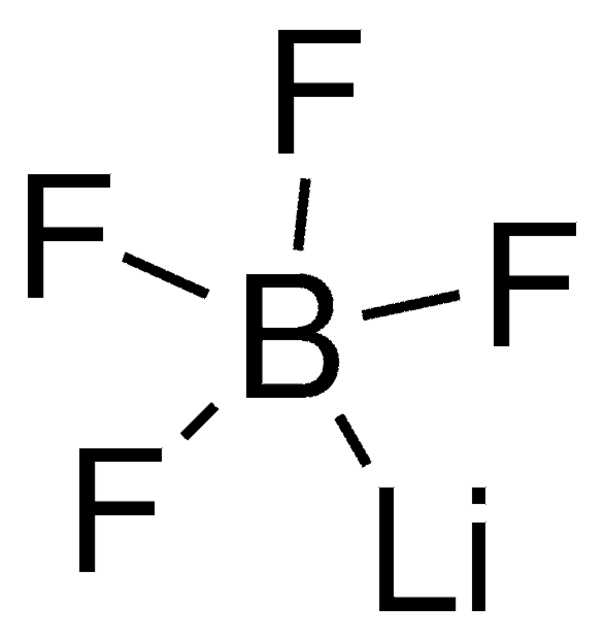
[Li+].F[B-](F)(F)F
InChI
1S/BF4.Li/c2-1(3,4)5;/q-1;+1
InChI 密鑰
UFXJWFBILHTTET-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
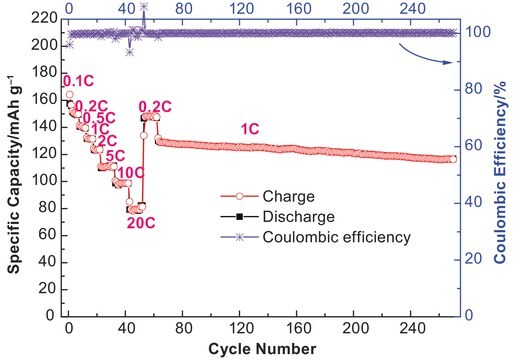
Lithium tetrafluoroborate (LiBF4), a lithium salt, exhibits high conductivity, low electrical charge transfer, moisture resistance, and good cyclic properties. It is suitable for a low-temperature electrolytic system. Its performance is comparable to the widely used LiPF6 system.
應用
Lithium tetrafluoroborate (LiBF4) finds application as electrolyte material in lithium ion batteries (LIBs). However, the presence of trace amount of water in LiBF4 causes its hydrolysis which leads to HF generation and destruction of the battery electrodes. Our LiBF4 contains < 100 ppm of water and is well suited for applications in LIBs.
注意
- These electrolyte solutions have extremely low water content; Please handle under inert and moisture free environment (glove box).
- Keep away from heat, sparks and flame. Avoid moisture, strong acid, strong alkalis, and oxidizing agents. Avoid skin and eye contact from product. Highly toxic and corrosive gases (HF) are generated by reaction with moisture os use only under inert gas atmosphere.
- Please handle under inert and moisture free environment.
Storage:
Keep containers away from heat, humidity, direct sunlight and ignition source. Keep containers tightly closed in a cool dry and well-ventilated place. Do not store with strong oxidizing agents.
法律資訊
Product of Ube Industries Ltd.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Muta. 2 - Skin Corr. 1B
儲存類別代碼
8B - Non-combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Doi T, et al.
Journal of the Electrochemical Society, 163, A2211-A2215 (2016)
Zuo X, et al.
Journal of the Electrochemical Society, 160, A1199-A1204 (2013)
Vanchiappan Aravindan et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(51), 14326-14346 (2011-11-25)
This paper presents an overview of the various types of lithium salts used to conduct Li(+) ions in electrolyte solutions for lithium rechargeable batteries. More emphasis is paid towards lithium salts and their ionic conductivity in conventional solutions, solid-electrolyte interface
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门