推荐产品
product name
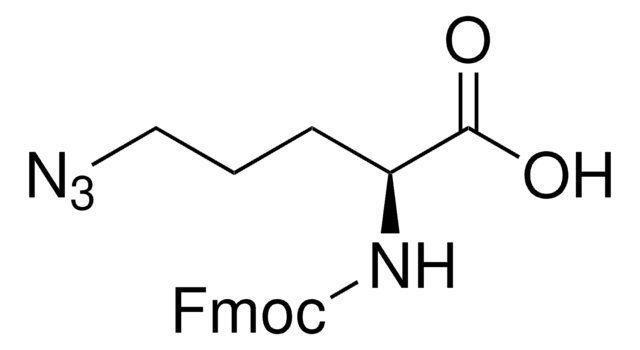
Fmoc-β-叠氮-Ala-OH, ≥98.0% (HPLC)
化驗
≥98.0% (HPLC)
形狀
lumps
光學活性
[α]/D -10.0±1.0°, c = 1 in DMF
反應適用性
reaction type: Fmoc solid-phase peptide synthesis
reaction type: click chemistry
雜質
15.3-16.5% nitrogen
60.1-62.5% carbon
應用
peptide synthesis
官能基
Fmoc
儲存溫度
2-8°C
SMILES 字串
OC(=O)[C@H](CN=[N+]=[N-])NC(=O)OCC1c2ccccc2-c3ccccc13
InChI
1S/C18H16N4O4/c19-22-20-9-16(17(23)24)21-18(25)26-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15-16H,9-10H2,(H,21,25)(H,23,24)/t16-/m0/s1
InChI 密鑰
ZITYCUDVCWLHPG-INIZCTEOSA-N
應用
Fmoc-β-azido-Ala-OH chloride is a general N-terminal protected reagent used in the solid phase peptide synthesis. Azido group allows it to undergo copper-mediated click chemistry reactions.
It can be used in:
It can be used in:
- Synthesis of α-N-acetylgalactosamine (α-GalNAc) linked antifreeze glycopeptides (AFGPs).
- Synthesis of stimuli-responsive multifunctional peptide gatekeepers for drug delivery applications.
- Synthesis of triazole-linked glycopeptides via Cu(I)-catalyzed 1,3-dipolar cycloaddition (CuAAC).
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Stimuli-responsive conformational conversion of peptide gatekeepers for controlled release of guests from mesoporous silica nanocontainers.
Lee, Jeonghun et al.
Journal of the American Chemical Society, 136(37), 12880-12883 (2014)
Synthesis of fish antifreeze neoglycopeptides using microwave-assisted ?click chemistry?.
Miller, Nicole et al.
Organic Letters, 11(11), 2409-2412 (2009)
Triazole phosphohistidine analogues compatible with the Fmoc-strategy.
McAllister, Tom E and Webb, Michael E
Organic & Biomolecular Chemistry, 10(20), 4043-4049 (2012)
Cu-catalyzed formation of triazole-linked glycoamino acids and application in chemoenzymatic peptide synthesis.
Kuijpers, Brian HM et al.
Organic Process Research & Development, 12(3), 503-511 (2008)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门