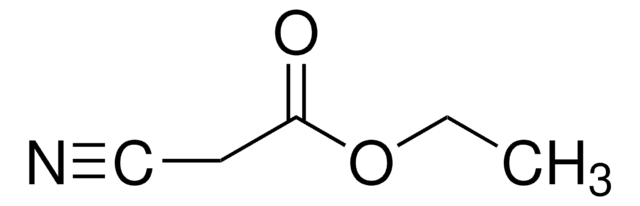
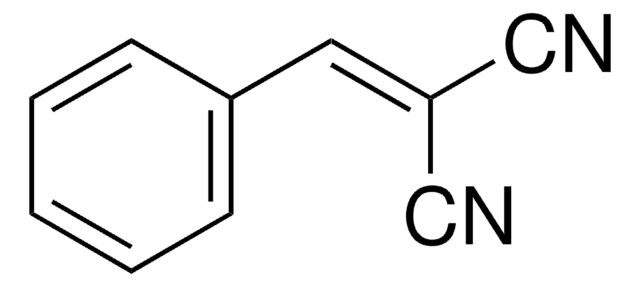
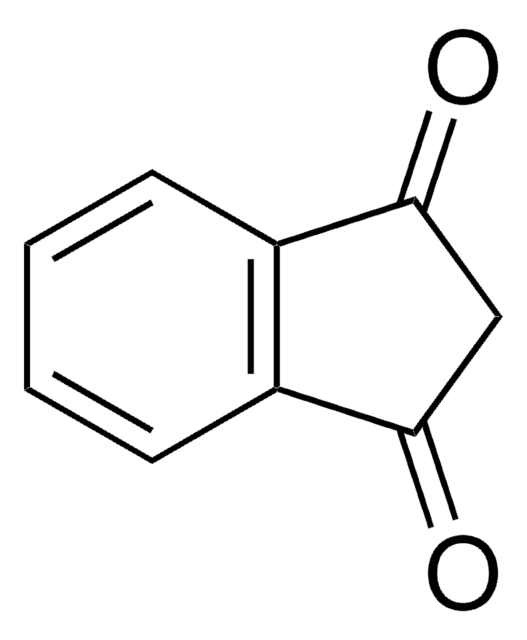
推荐产品
品質等級
化驗
≥99.0% (calculated, GC, KF)
形狀
liquid
品質
Arxada quality
製造商/商標名
Arxada AG
雜質
≤0.10% water
≤0.50% (E)-2-butenedinitrile
≤0.50% (Z)-2-butenedinitrile
≤0.50% butanedinitrile
bp
220 °C (lit.)
mp
30-32 °C (lit.)
密度
1.049 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
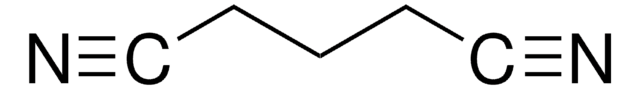
SMILES 字串
N#CCC#N
InChI
1S/C3H2N2/c4-2-1-3-5/h1H2
InChI 密鑰
CUONGYYJJVDODC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Malononitrile is an active methylene reagent useful for condensation reactions to synthesize various synthetic intermediates and heterocycles. It is extensively used in the Knoevenagel condensation with various aldehydes and ketones.
Some of the reactions where malononitrile is used as a reactant are:
Some of the reactions where malononitrile is used as a reactant are:
- Synthesis of 2-pyran-4-ylidene-malononitrile (PM) based red light emitting polymers.
- Synthesis of polysubstituted dihydropyridines.
- Synthesis of various chromene derivatives upon treating with salicylic aldehydes.
- Synthesis of triselenium dicyanide by treating it with selenium dioxide.
訊號詞
Danger
危險分類
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
186.8 °F - closed cup
閃點(°C)
86 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Triselenium dicyanide from malononitrile and selenium dioxide. One-pot synthesis of selenocyanates.
Kachanov, Andrey V et al.
Tetrahedron Letters, 45(23), 4461-4463 (2004)
Synthesis of polysubstituted dihydropyridines by four-component reactions of aromatic aldehydes, malononitrile, arylamines, and acetylenedicarboxylate.
Sun, Jing et al.
Organic Letters, 12(16), 3678-3681 (2010)
The Knoevenagel condensation reaction of aromatic aldehydes with malononitrile by grinding in the absence of solvents and catalysts.
Ren, Zhongjiao et al.
Synthetic Communications, 32(22), 3475-3479 (2002)
Synthesis and characterization of new red-emitting polyfluorene derivatives containing electron-deficient 2-pyran-4-ylidene- malononitrile moieties.
Peng, Qiang et al.
Macromolecules, 37(2), 260-266 (2004)
The condensation of salicylaldehydes and malononitrile revisited: synthesis of new dimeric chromene derivatives.
Costa, Marta et al.
The Journal of Organic Chemistry, 73(5), 1954-1962 (2008)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门
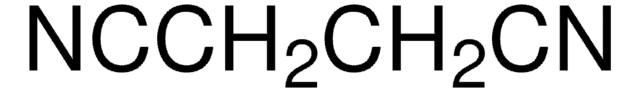
![2- [双(甲硫基)亚甲基]丙二腈 97%](/deepweb/assets/sigmaaldrich/product/structures/144/342/6a420594-3bce-4984-a8b7-5bf2a92d6a97/640/6a420594-3bce-4984-a8b7-5bf2a92d6a97.png)