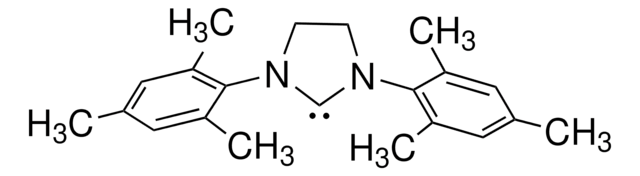
About This Item
推荐产品
品質等級
化驗
97%
形狀
solid
反應適用性
reagent type: ligand
mp
289-293 °C (lit.)
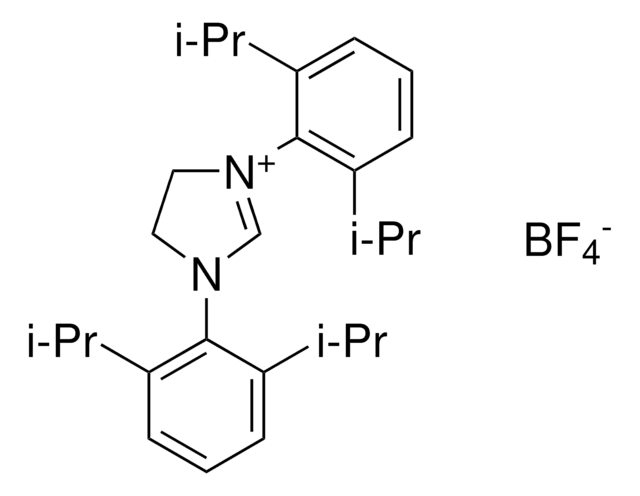
SMILES 字串
[Cl-].CC(C)c1cccc(C(C)C)c1N2CC[N+](=C2)c3c(cccc3C(C)C)C(C)C
InChI
1S/C27H39N2.ClH/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;/h9-14,17-21H,15-16H2,1-8H3;1H/q+1;/p-1
InChI 密鑰
LWPXTYZKAWSRIP-UHFFFAOYSA-M
應用
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
商品
A wide range of NHC ligands are commonly available which exhibit high activities.
Rapid progress in cross-coupling reactions of unactivated substrates catalyzed by metal complexes has transformed the chemical market-place through introduction of an extensive library of achiral and chiral phosphine ligands.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门





![氯[1,3-双(2,6-二异丙基苯基)咪唑-2-亚基]铜(I)](/deepweb/assets/sigmaaldrich/product/structures/199/763/44637b2e-b87c-42a3-abc3-3985b6cd7d5d/640/44637b2e-b87c-42a3-abc3-3985b6cd7d5d.png)