所有图片(1)
About This Item
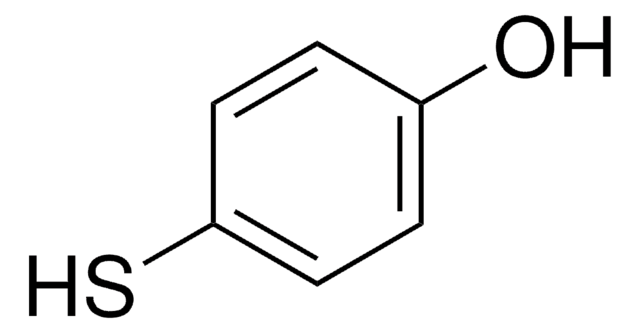
线性分子式:
HSC6H4CH2CO2H
CAS号:
分子量:
168.21
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
形狀
solid
mp
105-109 °C (lit.)
官能基
carboxylic acid
儲存溫度
2-8°C
SMILES 字串
OC(=O)Cc1ccc(S)cc1
InChI
1S/C8H8O2S/c9-8(10)5-6-1-3-7(11)4-2-6/h1-4,11H,5H2,(H,9,10)
InChI 密鑰
ORXSLDYRYTVAPC-UHFFFAOYSA-N
一般說明
4-巯基苯基乙酸(MPAA)是一种硫醇添加剂,广泛应用于自然化学偶联(NCL)反应,用于肽片段的选择性高效偶联。
應用
4-巯基苯基乙酸用于通过序列自然化学连接由双(2-磺酰乙基)氨基肽制备小泛素修饰肽。
可用于:
可用于:
- 树脂制备肽-α噻吩基酯,以在化学连接过程中化学合成多肽。
- 在一锅法中,采用天然化学连接和/或脱硫法去保护(乙酰胺基-甲基)半胱氨酸来制备多肽。
- 钯通过天然化学连接法促进N-端半胱氨酸去保护,以制备合成挑战性蛋白。
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Sunithi Gunasekera et al.
Frontiers in microbiology, 11, 168-168 (2020-03-11)
Can antimicrobial activity and peptide stability of alpha-helical peptides be increased by making them into dimers and macrocycles? Here, we explore that concept by using KR-12 as the starting point for peptide engineering. KR-12 has previously been determined as the
Lin Zhang et al.
Chemical science, 10(11), 3271-3280 (2019-04-19)
Targeted antibody blocking enables characterization of binding sites on immunoglobulin G (IgG), and can efficiently eliminate harmful antibodies from organisms. In this report, we present a novel peptide-denoted as a dual-functional conjugate of antigenic peptide and Fc-III mimetics (DCAF)-for targeted
Palladium mediated rapid deprotection of N-terminal cysteine under native chemical ligation conditions for the efficient preparation of synthetically challenging proteins
Jbara M, et al.
Journal of the American Chemical Society, 138(15), 5069-5075 (2016)
One-pot chemical synthesis of small ubiquitin-like modifier protein-peptide conjugates using bis (2-sulfanylethyl) amido peptide latent thioester surrogates
Boll E, et al.
Nature Protocols, 10(2), 269-269 (2015)
Daniel J Capon et al.
Proceedings of the Japan Academy. Series B, Physical and biological sciences, 87(9), 603-616 (2011-11-15)
There is a significant need for antibodies that can bind targets with greater affinity. Here we describe a novel strategy employing chemical semisynthesis to produce symmetroadhesins: antibody-like molecules having nonprotein hinge regions that are more flexible and extendible and are
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 653152-1G | 4061832732763 |
| 653152-5G | 4061832732770 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持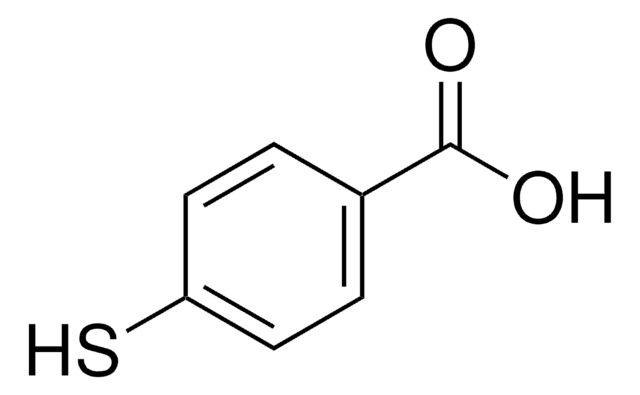



![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)