推荐产品
化驗
≥97.0% (T)
形狀
solid
官能基
carboxylic acid
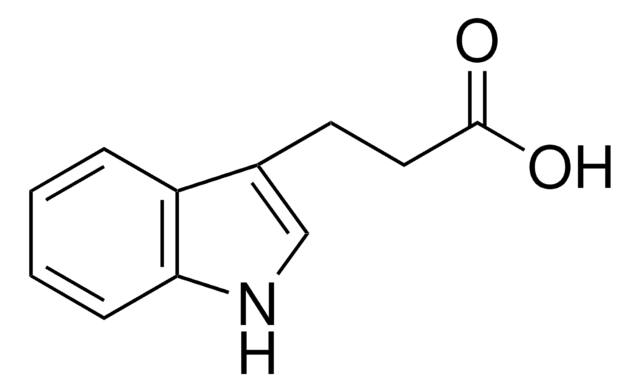
SMILES 字串
OC(=O)CCc1c[nH]c2ccccc12
InChI
1S/C11H11NO2/c13-11(14)6-5-8-7-12-10-4-2-1-3-9(8)10/h1-4,7,12H,5-6H2,(H,13,14)
InChI 密鑰
GOLXRNDWAUTYKT-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
吲哚-3-丙酸可以通过脱氨反应从色氨酸获得。
應用
反应物用于制备:
- 独脚金内酯的荧光类似物
- 抗肿瘤剂
- 黑皮质素受体配体
- 免疫抑制剂
- 丙肝病毒抑制剂
- 组胺H4受体激动剂
- NR2B/NMDA受体拮抗剂
- 治疗肥胖的 CB1 拮抗剂
- 抗细菌剂
- TGF-β受体结合抑制剂
生化/生理作用
被研究作为改善肝移植后灌注的辅助手段。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Wanguo Wei et al.
Bioorganic & medicinal chemistry letters, 19(24), 6926-6930 (2009-11-10)
New small molecule inhibitors of HCV were discovered by screening a small library of indoline alkaloid-type compounds. An automated assay format was employed which allowed identification of dimerization inhibitors of core, the capsid protein of the virus. These compounds were
Francis Giraud et al.
Bioorganic & medicinal chemistry letters, 20(17), 5203-5206 (2010-07-27)
N-aryl-3-(indol-3-yl)propanamides were synthesized and their immunosuppressive activities were evaluated. This study highlighted the promising potency of 3-[1-(4-chlorobenzyl)-1H-indol-3-yl]-N-(4-nitrophenyl)propanamide 15 which exhibited a significant inhibitory activity on murine splenocytes proliferation assay in vitro and on mice delayed-type hypersensitivity (DTH) assay in vivo.
Ragan, J. A.; et al.
Organic Process Research & Development, 13, 186-186 (2009)
Peng Xu et al.
Bioorganic & medicinal chemistry letters, 17(12), 3330-3334 (2007-04-27)
A series of novel acylide derivatives have been synthesized from erythromycin A via a facile procedure. By applying this procedure, cyclic carbonation to C-11,12 position, acylation to C-3 hydroxyl, and deprotection provided the desired acylides. These compounds showed antibacterial activity
Rosaria Gitto et al.
Bioorganic & medicinal chemistry, 17(4), 1640-1647 (2009-01-23)
A combined ligand-based and structure-based approach has previously allowed us to identify NR2B/NMDA receptor antagonists containing indole scaffold. In order to further explore the main structure activity relationships of this class of derivatives we herein report the design, synthesis and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门