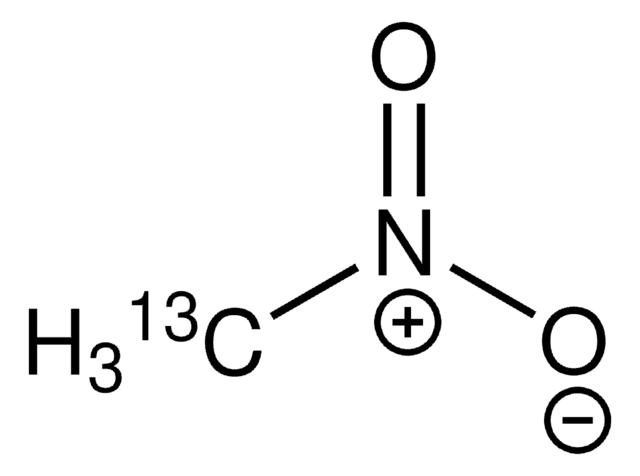
推荐产品
品質等級
化驗
97%
折射率
n20/D 1.478 (lit.)
bp
74 °C/19 mmHg (lit.)
密度
0.942 g/mL at 25 °C (lit.)
官能基
hydroxyl
SMILES 字串
OC1(CCCCC1)C=C
InChI
1S/C8H14O/c1-2-8(9)6-4-3-5-7-8/h2,9H,1,3-7H2
InChI 密鑰
ZXKHOVDDJMJXQP-UHFFFAOYSA-N
一般說明
1-Vinyl cyclohexanol, also known as 1-vinyl-1-cyclohexanol, is a tertiary allylic alcohol. It can be synthesized from cyclohexanone and vinyl chloride. It can undergo transition-metal-free tandem allylic borylation in the presence of B2pin [bis(pinacolato)diboron], Cs2CO3, THF and MeOH to yield triborated product. Pd-fullerite catalysts have been prepared which effectively catalyzes the hydrogenation of 1-ethynyl-1-cyclohexanol to 1-vinyl-1-cyclohexanol.
應用
1-Vinyl cyclohexanol may be used to synthesize:
- 1-vinyl-1-cyclohexene
- 1-vinyl-1-cyclohexylacrylate
- cyclohexylideneacetaldehyde
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
158.0 °F - closed cup
閃點(°C)
70 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Synthesis of spirocyclic butenolides by ring closing metathesis.
Albrecht U and Langer P.
Tetrahedron, 63(22), 4648-4654 (2007)
One-pot rapid low-cost synthesis of Pd-fullerite catalysts.
Chong LC, et al.
Journal of Materials Chemistry, 18(40), 4808-4813 (2008)
Lewis Acid Promoted Oxidative Rearrangement of Tertiary Allylic Alcohols with the PhIO/TEMPO System.
Vatele JM.
Synlett, 12, 1785-1788 (2008)
Transition-Metal-Free Borylation of Allylic and Propargylic Alcohols.
Miralles N, et al.
Angewandte Chemie (International Edition in English), 128(13), 4375-4379 (2016)
Dimerization of conjugated cyclodienes.
Suga K, et al.
Canadian Journal of Chemistry, 45(9), 933-937 (1967)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持