所有图片(1)
About This Item
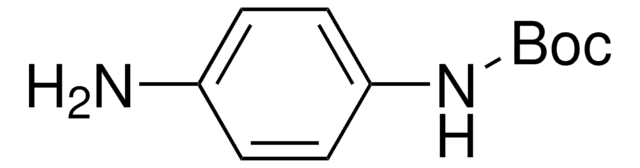
经验公式(希尔记法):
C11H16N2O2
CAS号:
分子量:
208.26
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
≥98.0% (HPLC)
反應適用性
reagent type: cross-linking reagent
官能基
Boc
amine
SMILES 字串
NC1=CC(NC(OC(C)(C)C)=O)=CC=C1
InChI
1S/C11H16N2O2/c1-11(2,3)15-10(14)13-9-6-4-5-8(12)7-9/h4-7H,12H2,1-3H3,(H,13,14)
InChI 密鑰
IEUIEMIRUXSXCL-UHFFFAOYSA-N
應用
N-Boc-m-phenylenediamine (tert-Butyl-3-aminophenylcarbamate) may be used in the preparation of:
- 5,5′-(propane-2,2-diyl)bis(N-(3-aminophenyl)-4-methyl-3-phenyl-1H-pyrrole-2-carboxamide)
- ethyl 4-[{3-[(tert-butoxycarbonyl)amino]phenyl}amino]-2-chloropyrimidine-5-carboxylate
- ethyl 4-(3-(tert-butoxycarbonyl)phenylamino)-2-(methylthio)pyrimidine-5-carboxylate
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Pyrimido [4,5-d] pyrimidin-4(1H)-one Derivatives as Selective Inhibitors of EGFR Threonine790 to Methionine790 (T790M) Mutants.
Xu T, et al.
Angewandte Chemie (Weinheim an der Bergstrasse, Germany), 125(32), 8545-8548 (2013)
Design, synthesis, and biological evaluation of novel conformationally constrained inhibitors targeting epidermal growth factor receptor threonine790? methionine790 mutant.
Chang S, et al.
Journal of Medicinal Chemistry, 55(6), 2711-2723 (2012)
Grigory V Kolesnikov et al.
Organic & biomolecular chemistry, 9(21), 7358-7364 (2011-09-07)
The design and synthesis of a neutral macrocyclic host that is capable of perrhenate and pertechnetate recognition is described. The anion affinities and underlying coordination modes were estimated by several experimental and theoretical methods including a new technique--reverse (99)Tc NMR
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![4-[(N-Boc)氨甲基]苯胺 97%](/deepweb/assets/sigmaaldrich/product/structures/341/155/530c425c-7e6e-435e-a28a-9d40b05b938a/640/530c425c-7e6e-435e-a28a-9d40b05b938a.png)