About This Item
推荐产品
product name
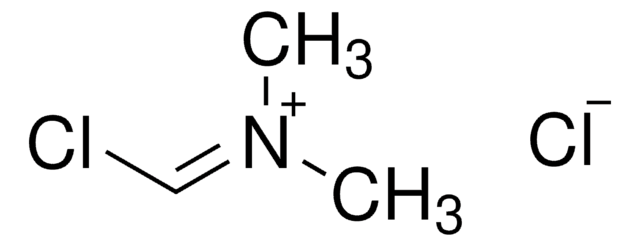
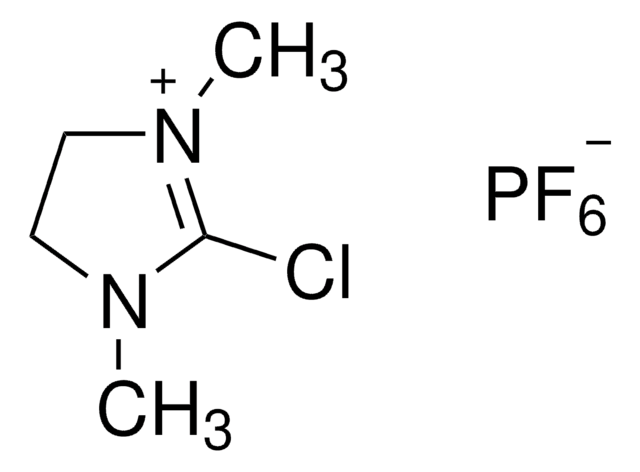
2-氯-1,3-二甲基氯化咪唑啉,
形狀
crystalline
品質等級
反應適用性
reaction type: Coupling Reactions
mp
133-140 °C (lit.)
應用
peptide synthesis
官能基
chloro
SMILES 字串
[Cl-].CN1CC[N+](C)=C1Cl
InChI
1S/C5H10ClN2.ClH/c1-7-3-4-8(2)5(7)6;/h3-4H2,1-2H3;1H/q+1;/p-1
InChI 密鑰
AEBBXVHGVADBHA-UHFFFAOYSA-M
應用
标记葡萄糖作为支链寡糖合成的中间体
荧光化学传感器
1,2-二胺作为共激活剂相关精氨酸甲基转移酶 1 的抑制剂
变构葡萄糖激酶激活剂
用于合成以下物质的反应物:
伯胺中的有机叠氮化物
aza-Henry 反应试剂
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
商品
N-Acylimidazoles were recognized in the early 1950s as reactive intermediates suitable for the acylation of amino compounds. The search for better coupling reagents than DCC led to the development of CDI (1,1’-carbonyldiimidazole) and related carbonylimidazoles.
N-Acylimidazoles were recognized in the early 1950s as reactive intermediates suitable for the acylation of amino compounds. The search for better coupling reagents than DCC led to the development of CDI (1,1’-carbonyldiimidazole) and related carbonylimidazoles.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门