所有图片(1)
About This Item
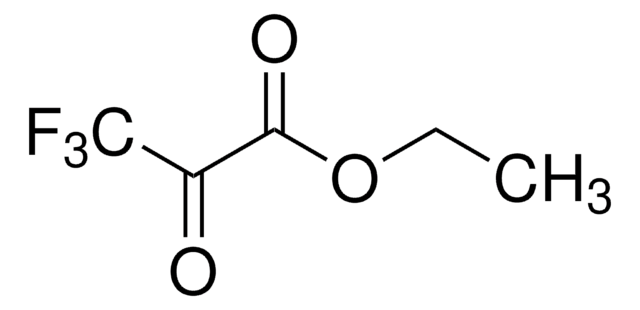
线性分子式:
CF3COCO2CH3
CAS号:
分子量:
156.06
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
97%
折射率
n20/D 1.332 (lit.)
bp
86 °C (lit.)
密度
1.529 g/mL at 25 °C (lit.)
官能基
ester
fluoro
ketone
SMILES 字串
COC(=O)C(=O)C(F)(F)F
InChI
1S/C4H3F3O3/c1-10-3(9)2(8)4(5,6)7/h1H3
InChI 密鑰
XGLLQDIWQRQROJ-UHFFFAOYSA-N
一般說明
Methyl 3,3,3-trifluoropyruvate is an alkyl 3,3,3-trifluoropyruvate that can be synthesized using hexafluoropropene-1,2-oxide (HFPO) as a starting material. It reacts with aromatic amines, benzylic manoamines and diamines to form the corresponding hemiaminals.
應用
Methyl 3,3,3-trifluoropyruvate may be used in the synthesis of:
- 2-hydroxy-2-trifluoromethylbutan-4-olides
- 2-(trifluoromethyl)butan-4-olides
- 4-trifluoromethyl-(2H)-pyridazin-3-ones
- methyl 3-methoxy-2-trifluoromethylacrylate
Fluorinated butanolides and butenolides: Part 8. 2-(Trifluoromethyl) butan-4-olides by synthesis from methyl 3, 3, 3-trifluoropyruvate as building block.
Paleta O, et al.
Journal of Fluorine Chemistry, 111(2), 175-184 (2001)
Fluorine-Sacrificial Cyclizations as an Access to 5-Fluoropyrazoles.
Volle JN and Schlosser M.
European Journal of Organic Chemistry, 2000(5), 823-828 (2000)
Methyl 3, 3, 3-trifluoropyruvate hemiaminals: Stability and transaminations.
Dolensky B, et al.
Journal of Fluorine Chemistry, 126(5), 745-751 (2005)
Fluorinated butanolides and butenolides: Part 9. Synthesis of 2-(trifluoromethyl) butan-4-olides by Wittig reaction using methyl 3, 3, 3-trifluoropyruvate.
Palecek J, et al.
Journal of Fluorine Chemistry, 113(2), 177-183 (2002)
Methyl 3,3,3-trifluoropyruvate: an improved procedure starting from hexafluoropropene-1, 2-oxide; identification of byproducts.
Dolensky B, et al.
Journal of Fluorine Chemistry, 115(1), 67- 74 (2002)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门