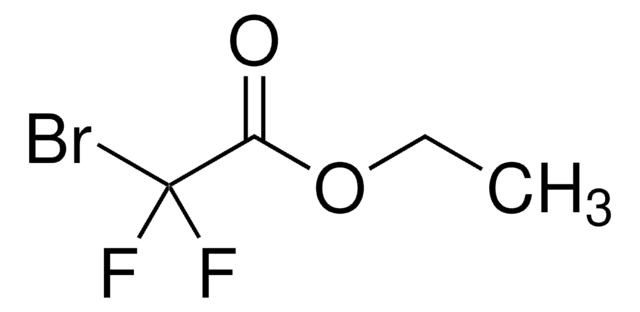
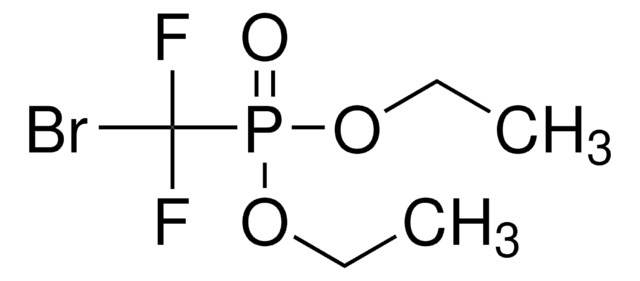
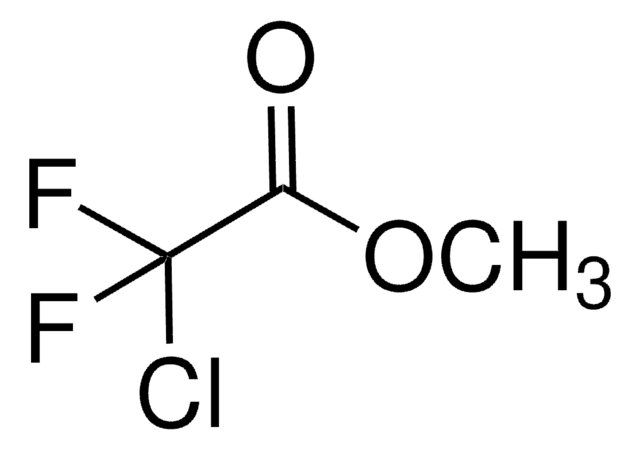
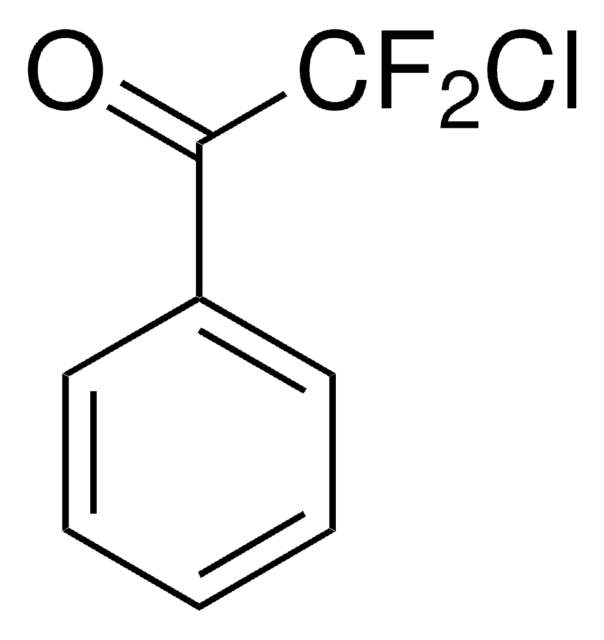
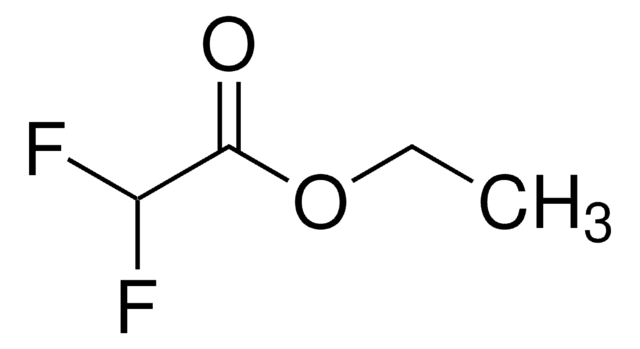
推荐产品
品質等級
化驗
98%
折射率
n20/D 1.358 (lit.)
bp
96-97.5 °C (lit.)
密度
1.252 g/mL at 25 °C (lit.)
官能基
chloro
ester
fluoro
SMILES 字串
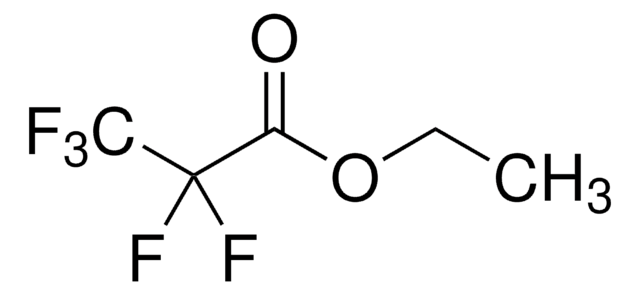
CCOC(=O)C(F)(F)Cl
InChI
1S/C4H5ClF2O2/c1-2-9-3(8)4(5,6)7/h2H2,1H3
InChI 密鑰
GVCAWQUJCHZRCB-UHFFFAOYSA-N
一般說明
Ethyl chlorodifluoroacetate (ECDFA) undergoes Reformatskii reaction with various aldehydes in DMF. It reacts with phenylacetylene to afford ethyl α,α−difluoro-4-phenyl-3-butenoates.
應用
Ethyl chlorodifluoroacetate (ECDFA) may be used as a starting reagent in the synthesis of 3-gem-difluoro-2-ethoxy allylic alcohols.
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
64.4 °F - closed cup
閃點(°C)
18 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Fluorine-containing organozinc reagents. IV.: Reformatskii-type reactions of chlorodifluoroacet1c acid derivatives.
Lang RW and Schaub B.
Tetrahedron Letters, 29(24), 2943-2946 (1988)
Synthesis of 3-gem-difluoro-2-ethoxy allylic alcohols from ethyl chlorodifluoroacetate.
Begue J-P, et al.
Tetrahedron Letters, 35(3), 6097-6100 (1994)
A theoretical investigation on the kinetics and reactivity of the gas-phase reactions of ethyl chlorodifluoroacetate with OH radical and Cl atom at 298 K.
Mishra BK, et al.
Structural Chemistry, 25(2), 463-470 (2014)
Wadih Ghattas et al.
The Journal of organic chemistry, 71(22), 8618-8621 (2006-10-27)
An efficient preparation of pure ethyl Z- and E-alpha,alpha-difluoro-4-phenyl-3-butenoate 1a and 1b together with the corresponding acids 2a and 2b is described. The procedures involve stereocontrolled additions of *CF2CO2Et to phenylacetylene or beta-bromostyrene. Compound 1a is easily obtained by addition
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门