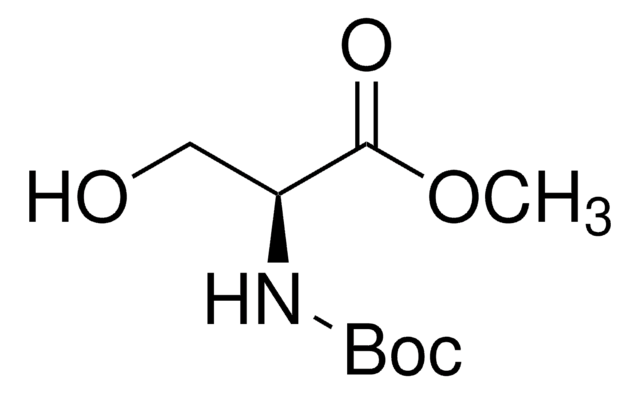
推荐产品
品質等級
化驗
95%
反應適用性
reaction type: solution phase peptide synthesis
bp
170 °C/0.01 mmHg (lit.)
mp
41-43 °C (lit.)
應用
peptide synthesis
SMILES 字串
COC(=O)[C@H](CO)NC(=O)OCc1ccccc1
InChI
1S/C12H15NO5/c1-17-11(15)10(7-14)13-12(16)18-8-9-5-3-2-4-6-9/h2-6,10,14H,7-8H2,1H3,(H,13,16)/t10-/m0/s1
InChI 密鑰
CINAUOAOVQPWIB-JTQLQIEISA-N
應用
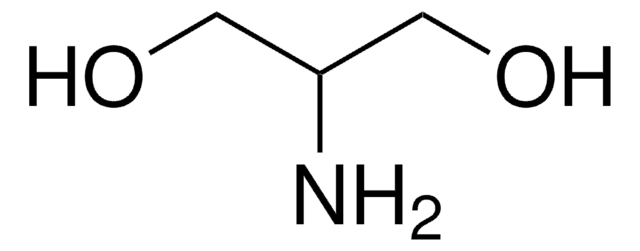
该保护性丝氨酸已作为 Garner 醛的 Cbz 类似物、2,3-二氨基丙醇和 2-氨基-1,3-丙二醇的光学活性衍生物的原料。
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
Demirci, F. et al.
Synthesis, 189-189 (1996)
Kang, M. et al.
The Journal of Organic Chemistry, 61, 5528-5528 (1996)
Monache, G.D. et al.
Synthesis, 1155-1155 (1995)
James A. Marshall et al.
The Journal of organic chemistry, 61(2), 581-586 (1996-01-26)
The aminoheptose destomic acid (3.5) and the aminooctose lincosamine (6.8) were synthesized in protected form by parallel sequences starting from the oxazolidine derivatives 2.4 and 5.1 of N-CBz serinal and N-BOC threoninal. The parallel sequences feature BF(3)-promoted addition of the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门