所有图片(2)
About This Item
经验公式(希尔记法):
C8H7Br
CAS号:
分子量:
183.05
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
95%
折射率
n20/D 1.59 (lit.)
bp
90 °C/10.5 mmHg (lit.)
密度
1.45 g/mL at 25 °C (lit.)
官能基
bromo
SMILES 字串
BrC1Cc2ccccc12
InChI
1S/C8H7Br/c9-8-5-6-3-1-2-4-7(6)8/h1-4,8H,5H2
InChI 密鑰
AYNXHFRDABNHRX-UHFFFAOYSA-N
一般說明
1-Bromobenzocyclobutene is a useful synthon. It has important applications in organometallic methodology. Reaction between cycloheptatriene, bromoform, potassium carbonate and 18-crown-6 at 140°C yields 1-bromobenzocyclobutene.
應用
1-Bromobenzocyclobutene may be used in the synthesis of following compounds:
- five-membered zirconacycles
- benzocyclobutenol and benzocyclobutenone
- 2,3-dimethoxyprotoberberinium bromide, via reaction with 3,4-dihydro-6,7-dimethoxyisoquinoline
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
204.8 °F - closed cup
閃點(°C)
96.00 °C - closed cup
個人防護裝備
Eyeshields, Gloves
T V V Ramakrishna et al.
Organic letters, 5(6), 877-879 (2003-03-14)
[reaction: see text] Commercially available 1-bromobenzocyclobutene is a potentially useful synthon particularly with the application of organometallic methodology. Here we show that it is readily converted into Cp(2)Zr(benzocyclobutadiene), which couples with alkynes or nitriles giving five-membered zirconacycles. Treatment of these
1-Bromobenzocyclobutene: a convenient entry into the benzocyclobutene ring system.
DeCamp MR and Viscogliosi LA.
The Journal of Organic Chemistry, 46(19), 3918-3920 (1981)
Condensed Cyclobutane Aromatic Compounds. IX. Benzocyclobutenol and Benzocyclobutenone.
Cava MP and Muth K.
Journal of the American Chemical Society, 82(3), 652-654 (1960)
Studies on the syntheses of heterocyclic compounds-DXLV: An alternative synthesis of the protoberberine ring system.
Kametani T, et al.
Tetrahedron, 30(9), 1043-1046 (1974)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门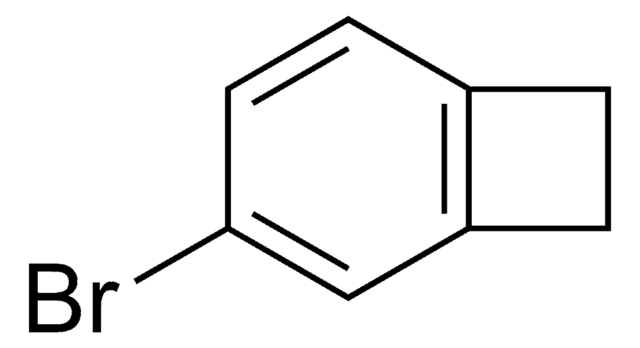
![BICYCLO[4.2.0]OCTA-1,3,5-TRIEN-7-ONE AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/105/021/61bb8f7c-e86a-48bf-8d63-aa68ad3c21df/640/61bb8f7c-e86a-48bf-8d63-aa68ad3c21df.png)