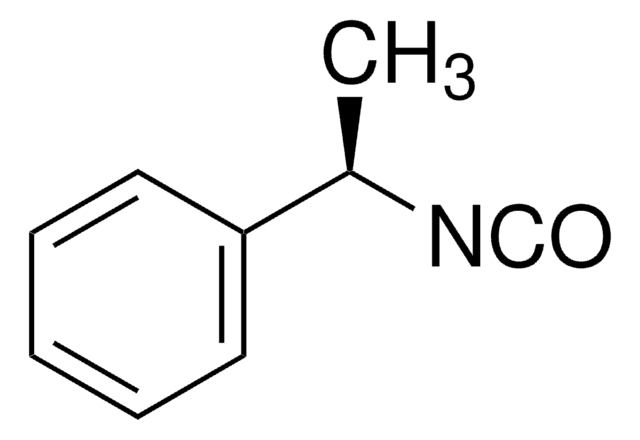
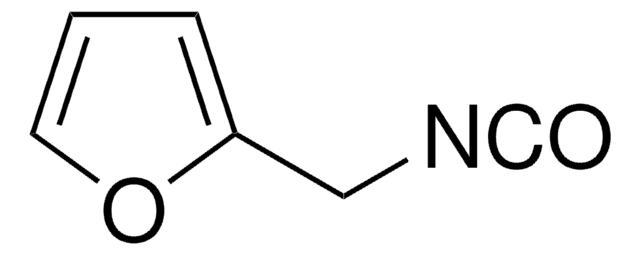
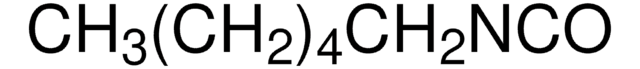推荐产品
品質等級
化驗
98%
折射率
n20/D 1.522 (lit.)
bp
210 °C (lit.)
密度
1.063 g/mL at 25 °C (lit.)
官能基
amine
isocyanate
phenyl
SMILES 字串
O=C=NCCc1ccccc1
InChI
1S/C9H9NO/c11-8-10-7-6-9-4-2-1-3-5-9/h1-5H,6-7H2
InChI 密鑰
HACRKYQRZABURO-UHFFFAOYSA-N
訊號詞
Danger
危險分類
Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Resp. Sens. 1 - Skin Corr. 1A - Skin Sens. 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 2
閃點(°F)
212.0 °F - closed cup
閃點(°C)
100 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Andreas Habel et al.
Journal of chromatography. A, 1165(1-2), 182-190 (2007-08-19)
1-phenylethyl isocyanate (1-PEIC), a chiral derivatisation reagent for the resolution of secondary alcohols is a powerful tool to determine the configuration and enantiomeric excess of medium- to long-chain secondary alcohols by capillary gas chromatography. The separation of 1-phenylethylcarbamates (1-PECs) of
J Gal et al.
Drug metabolism and disposition: the biological fate of chemicals, 9(6), 557-560 (1981-11-01)
Chiral secondary alcohols were treated with (S)-(-)-1-phenylethyl isocyanate. For each racemic alcohol, the resulting diastereomeric urethane derivatives were resolved on flexible fused-silica capillary GLC columns with retention times of 15 min or less. Derivatization of individual enantiomers showed that the
Silica gel high-performance liquid chromatography for the simultaneous determination of propranolol and 4-hydroxypropranolol enantiomers after chiral derivatization.
M J Wilson et al.
Journal of chromatography, 310(2), 424-430 (1984-10-12)
E Albano et al.
Hepatology (Baltimore, Md.), 23(1), 155-163 (1996-01-01)
We have previously shown that the treatment with diallyl sulfide (DAS) and phenylethyl isothiocyanate (PIC) of rats receiving ethanol in the alcohol tube-feeding model effectively suppressed the induction of cytochrome P4502E1 (CYP2E1) by ethanol. Here we report that rat treatment
A Adesida et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 34(4), 385-392 (1996-04-01)
Mercapturic acid pathway metabolites of phenylethyl isothiocyanate inhibited the growth of human leukaemia 60 (HL60) cells in vitro. The adduct with L-cysteine, S-(N-phenylethylthiocarbamoyl)cysteine, was the most potent with strong antileukaemic activity: the median growth inhibitory concentration (GC50) value was 336
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持