About This Item
推荐产品
品質等級
化驗
97%
形狀
solid
反應適用性
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
core: palladium
mp
280 °C (dec.) (lit.)
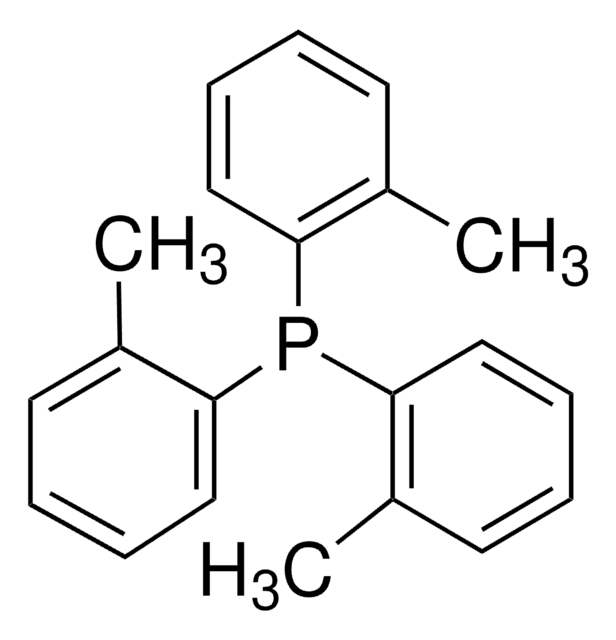
SMILES 字串
Cl[Pd]Cl.Cc1ccccc1P(c2ccccc2C)c3ccccc3C.Cc4ccccc4P(c5ccccc5C)c6ccccc6C
InChI
1S/2C21H21P.2ClH.Pd/c2*1-16-10-4-7-13-19(16)22(20-14-8-5-11-17(20)2)21-15-9-6-12-18(21)3;;;/h2*4-15H,1-3H3;2*1H;/q;;;;+2/p-2
InChI 密鑰
OTYPIDNRISCWQY-UHFFFAOYSA-L
一般說明
應用
- 由甲醇三丁锡和乙酸烯醇酯与芳基溴化物原位制备三丁基锡烯酸酯的反应。
- 芳基溴化物与乙酸乙烯酯的偶联反应。
- 芳基溴化物与 2-(三丁基锡烷基)乙酸乙酯的 Negishi-Reformatsky 偶联反应。
- 通过 Heck 反应合成(E)3-(7-吲哚基)-2-甲基丙烯酸甲酯。
- 咪唑并嘧啶衍生物的合成。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
商品
A variety of transition-metal catalysts for the Suzuki coupling reaction are now available in our catalog. The majority of these catalysts are palladium- and nickelbased, typically utilizing phosphine-derived ligands.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门



![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)