所有图片(3)
About This Item
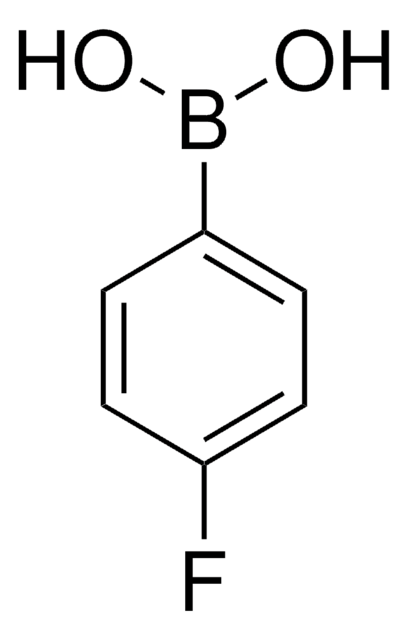
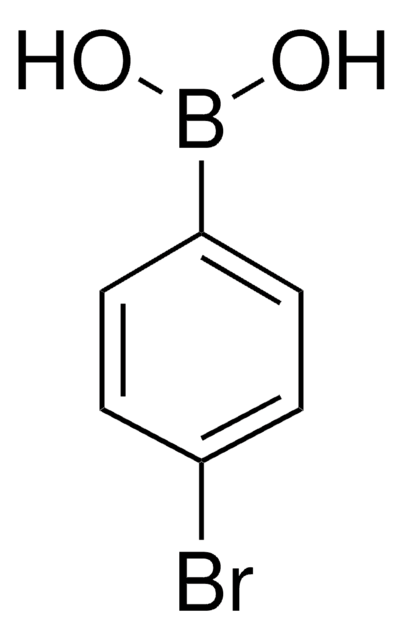
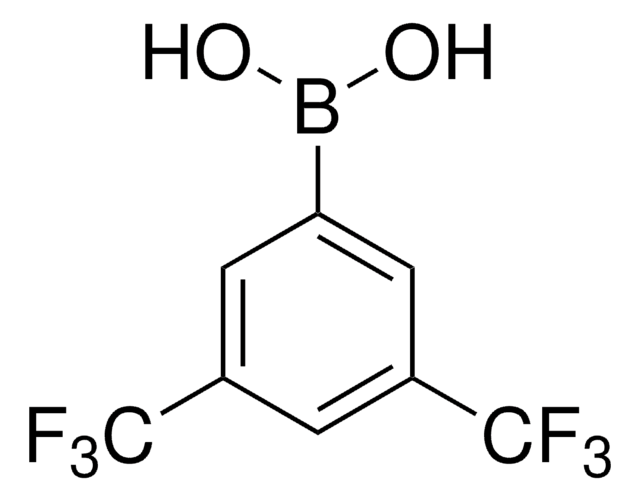
线性分子式:
CF3C6H4B(OH)2
CAS号:
分子量:
189.93
Beilstein:
3544189
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥95.0%
mp
245-250 °C (lit.)
官能基
fluoro
SMILES 字串
OB(O)c1ccc(cc1)C(F)(F)F
InChI
1S/C7H6BF3O2/c9-7(10,11)5-1-3-6(4-2-5)8(12)13/h1-4,12-13H
InChI 密鑰
ALMFIOZYDASRRC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
4-三氟甲基苯基硼酸可用作以下物质的反应物:
它也可作为反应物合成:
- 位点选择Suzuki-Miyaura交叉偶联反应
- 钯催化的直接芳基化反应
- 串联型Pd(II) 催化的氧化Heck反应和分子内C-H酰胺化序列
- 钌催化的直接芳基化
- 无配体铜催化的偶联反应
- 胺化和结合物加成反应
- 通过Suzuki-Miyaura和Sonogashira交叉偶联反应进行区域选择性芳基化和炔基化
- 铑催化不对称1,4-加成反应
- 铜催化的硝化反应
- 区域选择性Suzuki-Miyaura偶联和串联钯催化的分子内氨基羰基化和环化
- 钯催化的烯丙醇的烯丙基化反应
- 以铜交换氟磷灰石为催化剂进行咪唑和胺的N-芳基化反应
它也可作为反应物合成:
- 可打印电子设备用噻唑衍生物
- 三联苯苯并咪唑类微管蛋白聚合抑制剂
- 芳基酮(通过与酰氯发生交叉偶联反应)
其他說明
含有不定量的酸酐。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Efficient synthesis of arylated coumarins by site-selective Suzuki-Miyaura cross-coupling reactions of the bis(triflate) of 4-methyl-5,7-dihydroxy-coumarin
Eleya, N.; et al.
Synlett, 23, 223-226 (2012)
S Van Mierloo et al.
Magnetic resonance in chemistry : MRC, 50(5), 379-387 (2012-04-18)
Four 2,5-bis(5-aryl-3-hexylthiophen-2-yl)thiazolo[5,4-d]thiazole derivatives have been synthesized and thoroughly characterized. The extended aromatic core of the molecules was designed to enhance the charge transport characteristics, and solubilizing hexyl side chains were introduced on the thiophene subunits to enable possible integration of
Viktor O Iaroshenko et al.
Organic & biomolecular chemistry, 10(15), 2955-2959 (2012-03-10)
A facile synthetic approach for the synthesis of 1,8-naphthyridine-4(1H)-one derivatives via a catalyst free and Pd-supported tandem amination sequence is developed and described. In a case of aliphatic amines reaction proceeds in a catalyst free mode, however anilines demand Pd-supported
Tetrahedron, 62, 11675-11675 (2006)
M Lakshmi Kantam et al.
The Journal of organic chemistry, 71(25), 9522-9524 (2006-12-02)
N-Arylation of imidazoles and amines with arylboronic acids was accomplished with copper-exchanged fluorapatite (CuFAP) in methanol at room temperature. The products N-arylimidazoles and N-arylamines were isolated in good to excellent yields. A variety of arylboronic acids were converted to the
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门