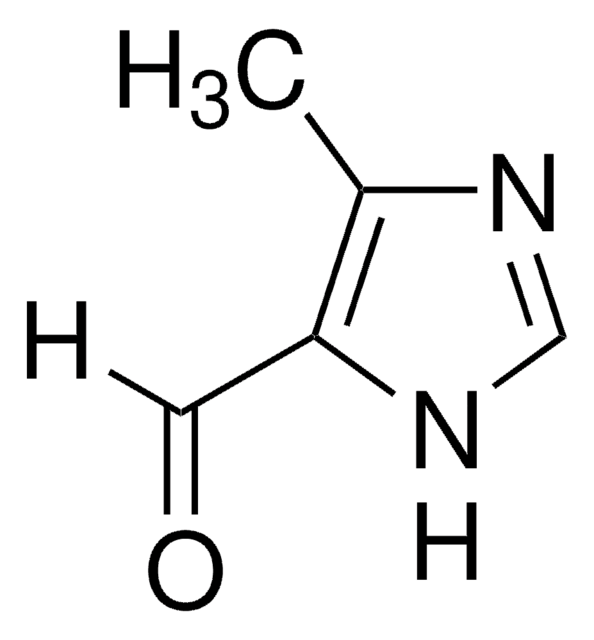
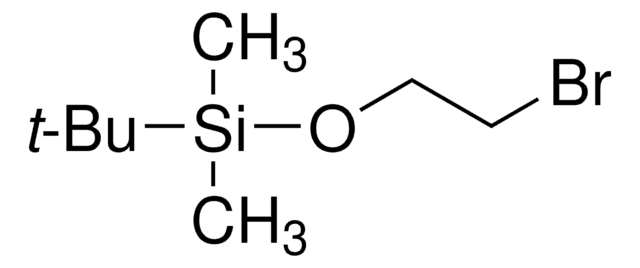
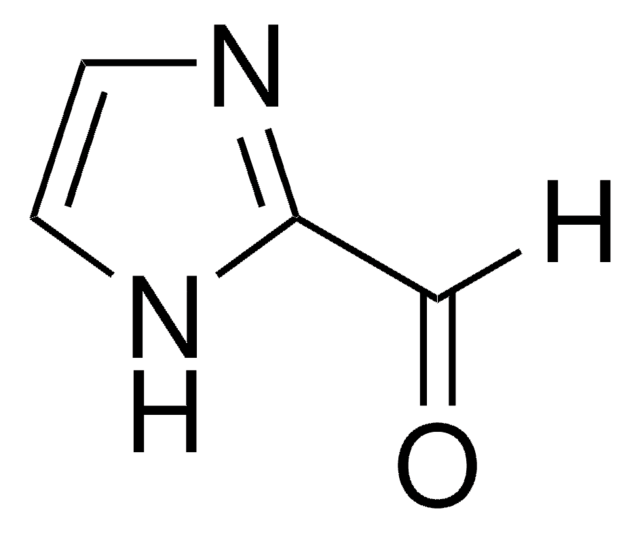
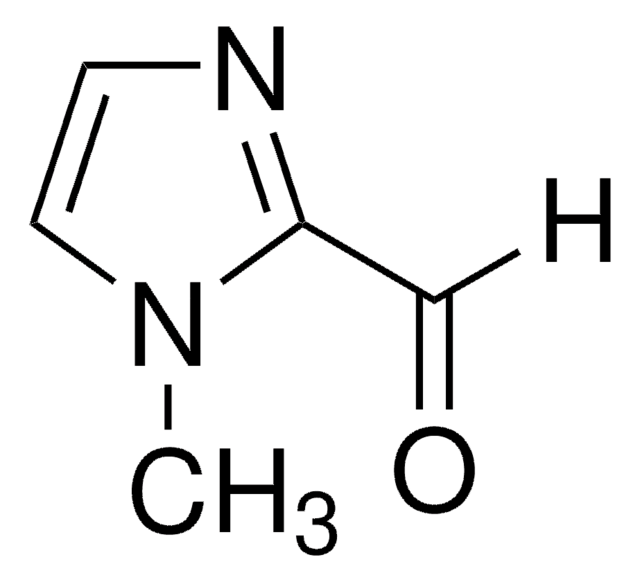
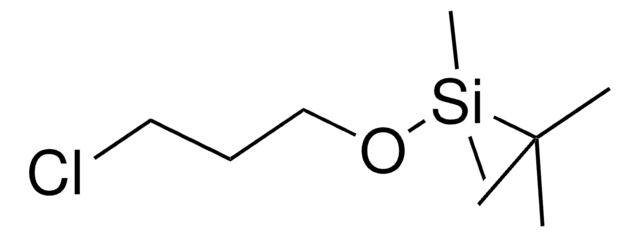
429066
(3-溴丙氧基)-叔丁基二甲基硅烷
97%
别名:
(3-Bromopropoxy)(1,1-dimethylethyl)dimethylsilane, 1-((tert-Butyldimethylsilyl)oxy)-3-bromopropane, 1-Bromo-3-(tert-butyldimethylsiloxy)propane, 1-Bromo-3-[(tert-butyldimethylsilanyl)oxy]propane, 1-Bromo-3-[(tert-butyldimethylsilyl)oxy]propane, 3-((tert-Butyldimethylsilyl)oxy)-1-bromopropane
登录查看公司和协议定价
所有图片(1)
About This Item
线性分子式:
Br(CH2)3OSi(CH3)2C(CH3)3
CAS号:
分子量:
253.25
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
一般說明
(3-Bromopropoxy)-tert-butyldimethylsilane is a bromo silyl ether.
應用
(3-Bromopropoxy)-tert-butyldimethylsilane may be used to introduce propanol functionality to many pharmaceuticals.
It may be used as an alkylating agent in the synthesis of the following:
It may be used as an alkylating agent in the synthesis of the following:
- N-[2-[N-[3-(tert-butyldimethylsilyloxy)propyl]-N-ethylamino]ethyl]phthalimide
- O-(3-tert-butyldimethylsilyloxypropyl)-N-(tert-butoxycarbonyl)-L-tyrosine methyl ester
- tert-butyldimethyl-[3-(3-methyl-2-nitrophenoxy)propoxy]silane
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
185.0 °F - closed cup
閃點(°C)
85 °C - closed cup
其他客户在看
Synthesis of O-(3-[18F] Fluoropropyl)-L-tyrosine (L-[18F] FPT) and its biological evaluation in 9L tumor bearing rat.
Moon BS, et al.
Bull. Korean Chem. Soc., 26(1), 91-96 (2005)
Ramani R Ranatunge et al.
Journal of medicinal chemistry, 47(9), 2180-2193 (2004-04-16)
The synthesis of a series of novel pyrazoles containing a nitrate (ONO(2)) moiety as a nitric oxide (NO)-donor functionality is reported. Their COX-1 and COX-2 inhibitory activities in human whole blood are profiled. Our data demonstrate that pyrazole ring substituents
A concise total synthesis of (+/-)-vigulariol.
J Stephen Clark et al.
Angewandte Chemie (International ed. in English), 46(3), 437-440 (2006-12-06)
Han-Cheng Zhang et al.
Bioorganic & medicinal chemistry letters, 14(12), 3245-3250 (2004-05-20)
A novel series of acyclic 3-(7-azaindolyl)-4-(aryl/heteroaryl)maleimides was synthesized and evaluated for activity against GSK-3beta and selectivity versus PKC-betaII, as well as a broad panel of protein kinases. Compounds 14 and 17c potently inhibited GSK-3beta (IC(50)=7 and 26 nM, respectively) and
Synthesis, radioiodination and in vivo screening of novel potent iodinated and fluorinated radiotracers as melanoma imaging and therapeutic probes.
Maisonial A, et al.
European Journal of Organic Chemistry, 63, 840-853 (2013)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门