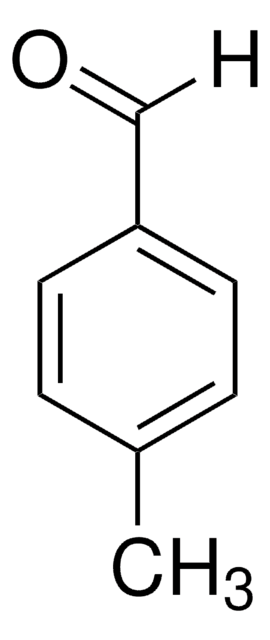
推荐产品
一般說明
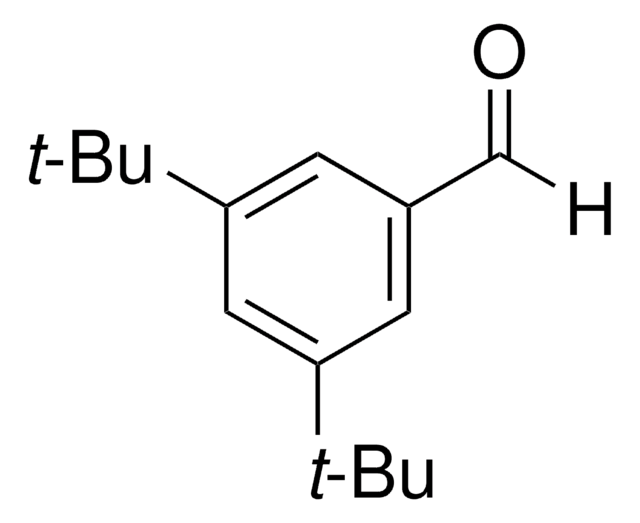
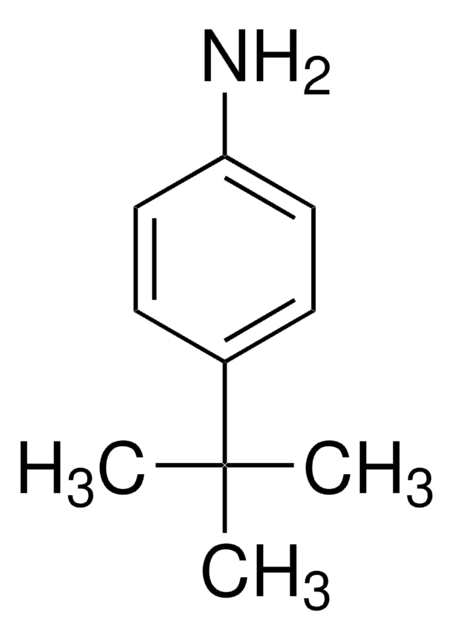
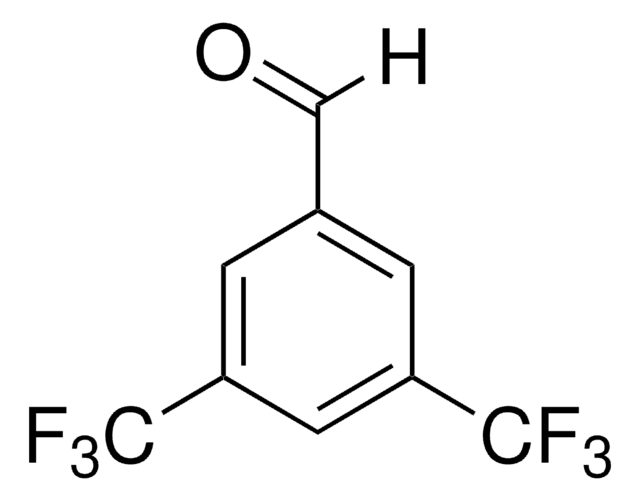
4-叔丁基苯甲醛是合成医药、染料、香料和香料的重要中间体。据报告,它是在冰醋酸中用过氧化氢部分氧化 4- 叔 -丁基甲苯过程中形成的,由溴离子与乙酸钴 (II) 或乙酸铈 (III) 结合催化。在基体辅助激光解吸电离 (MALDI) 室中进行了 4- 叔 丁基苯胺和 4- 叔 丁基苯甲醛在乙醇中的 Schiff 碱反应,对生成的亚胺进行了实时检测。
應用
4- 叔丁基苯甲醛适用于动力学研究,以评价 4-取代苯甲醛抑制蘑菇酪氨酸酶的动力学常数 (KI)。
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Aquatic Acute 1 - Repr. 2 - Skin Sens. 1
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
213.8 °F - closed cup
閃點(°C)
101 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Leon G A van de Water et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(28), 8037-8044 (2007-07-12)
The partial oxidation of 4-tert-butyltoluene to 4-tert-butylbenzaldehyde by hydrogen peroxide in glacial acetic acid, catalyzed by bromide ions in combination with cobalt(II) acetate or cerium(III) acetate, has been studied in detail. Based on the observed differences in reaction rates and
Monica Brivio et al.
Lab on a chip, 5(4), 378-381 (2005-03-26)
The integration of a monitoring port along the microfluidic path of a MALDI-chip integrated device is described. Optimization of the microreactor design allows longer reaction and measuring times. The Schiff base reaction between 4-tert-butylaniline (1) and 4-tert-butylbenzaldehyde (2) in ethanol
M Jiménez et al.
Journal of agricultural and food chemistry, 49(8), 4060-4063 (2001-08-22)
A kinetic study of the inhibition of mushroom tyrosinase by 4-substituted benzaldehydes showed that these compounds behave as classical competitive inhibitors, inhibiting the oxidation of L-3,4-dihydroxyphenylalanine (L-DOPA) by mushroom tyrosinase (o-diphenolase activity). The kinetic parameter (K(I)) characterizing this inhibition was
Chao-Bin Xue et al.
Bioorganic & medicinal chemistry, 15(5), 2006-2015 (2007-01-30)
Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-quinones. Inhibition of PO may provide a basis for novel environmentally friendly
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门