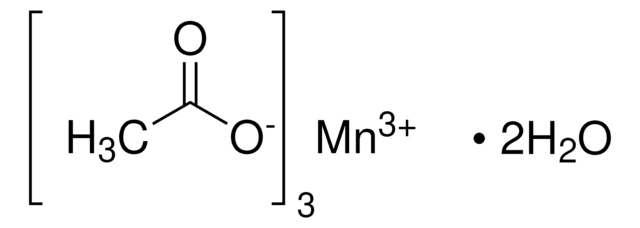
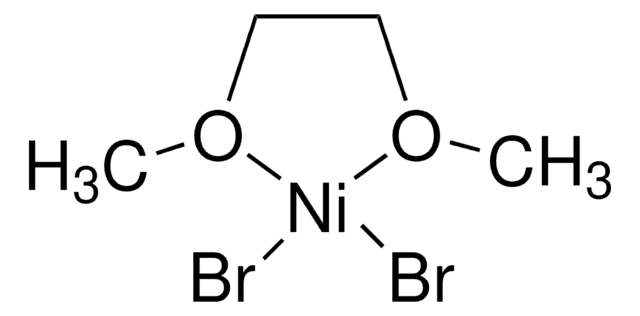
About This Item
推荐产品
形狀
liquid
反應適用性
reaction type: C-C Bond Formation
濃度
0.5 M in toluene
密度
0.927 g/mL at 25 °C
SMILES 字串
[CH]1[CH][CH][CH][CH]1.[CH]2[CH][CH][CH][CH]2.C[Al](C)C[Ti]Cl
InChI
1S/2C5H5.2CH3.CH2.Al.ClH.Ti/c2*1-2-4-5-3-1;;;;;;/h2*1-5H;2*1H3;1H2;;1H;/q;;;;;;;+1/p-1
InChI 密鑰
QEJAQNUJXFLWSP-UHFFFAOYSA-M
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- 使叶绿素衍生物的羰基转化为相应的环外亚甲基(亚乙烯基)。
- 参与由3-OH乙二醇酯合成β-C糖苷的反应。
- 作为多功能的亚甲基试剂参与酮和醛转化为烯烃的反应。它可与受阻酮进行温和的反应,并可使酯转化为乙烯基醚。
- 使醛烯化。
- 使具有高非对映选择性的手性多羟基酮亚甲基化。
包裝
法律資訊
訊號詞
Danger
危險分類
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1B - STOT RE 2 - STOT SE 3
標靶器官
Central nervous system, Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
39.2 °F - closed cup
閃點(°C)
4 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
商品
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门