所有图片(1)
About This Item
经验公式(希尔记法):
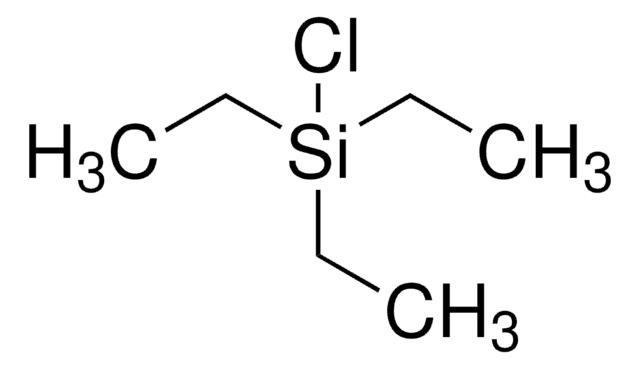
C13H25NSi
CAS号:
分子量:
223.43
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
95%
形狀
liquid
折射率
n20/D 1.492 (lit.)
bp
78 °C/0.4 mmHg (lit.)
密度
0.904 g/mL at 25 °C (lit.)
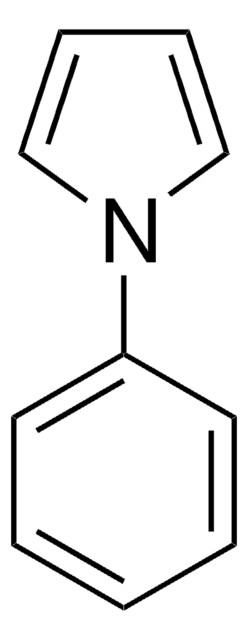
SMILES 字串
CC(C)[Si](C(C)C)(C(C)C)n1cccc1
InChI
1S/C13H25NSi/c1-11(2)15(12(3)4,13(5)6)14-9-7-8-10-14/h7-13H,1-6H3
InChI 密鑰
FBQURXLBJJNDBX-UHFFFAOYSA-N
一般說明
1-(Triisopropylsilyl)pyrrole (TISP), a heterocyclic building block, is a pyrrole derivative. TISP has been reported to generate pyrrolic cation radicals during cyclovoltammetric studies, via electroreduction. It participates in various electrophilic substitution reactions specifically at β-position, via reaction with various electrophilic reagents (Br+, I+,NO2+,etc).
應用
用于全氟烷基化和 Vilsmeier 甲酰化反应。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
224.6 °F - closed cup
閃點(°C)
107 °C - closed cup
N-(triisopropylsilyl) pyrrole. A progenitor" par excellence" of 3-substituted pyrroles.
Bray BL, et al.
The Journal of Organic Chemistry, 55(26), 6317-6328 (1990)
Daniel A Harki et al.
Biochemistry, 41(29), 9026-9033 (2002-07-18)
Synthetic small molecules that promote viral mutagenesis represent a promising new class of antiviral therapeutics. Ribavirin is a broad-spectrum antiviral nucleoside whose antiviral mechanism against RNA viruses likely reflects the ability of this compound to introduce mutations into the viral
Observation of the cation radicals of pyrrole and of some substituted pyrroles in fast-scan cyclic voltammetry. Standard potentials and lifetimes.
Andrieux CP, et al.
Journal of the American Chemical Society, 112(6), 2439-2440 (1990)
Reaction of pyrroles with ethyl 2-nitroso-and 2-azo-propenoates, and with ethyl cyanoformate N-oxide: a comparison of the reaction pathways.
Gilchrist TL and Lemos A.
Journal of the Chemical Society. Perkin Transactions 1, 13, 1391-1395 (1993)
Synthesis, Structure, and Deoxyribonucleic Acid Sequencing with a Universal Nucleoside: 1-(2'-Deoxy-. beta.-D-ribofuranosyl)-3-nitropyrrole.
Bergstrom DE, et al.
Journal of the American Chemical Society, 117(4), Synthesis-Synthesis (1999)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门