所有图片(1)
About This Item
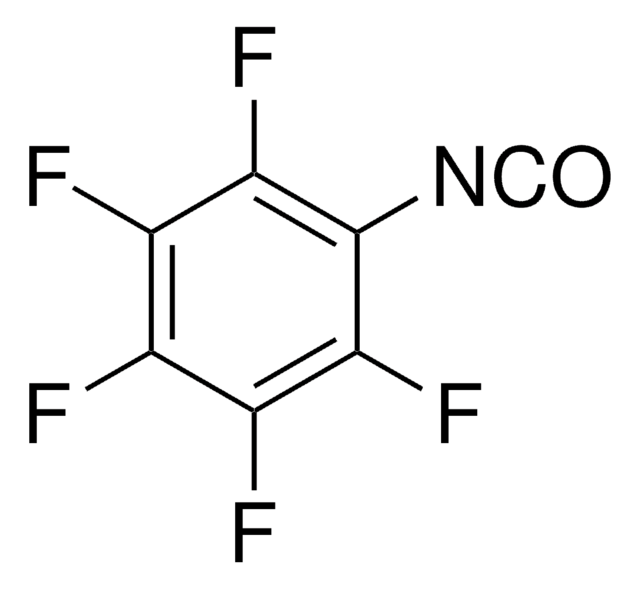
线性分子式:
(CF3)2C6H3NCO
CAS号:
分子量:
255.12
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
形狀
liquid
折射率
n20/D 1.43 (lit.)
密度
1.476 g/mL at 25 °C (lit.)
官能基
fluoro
isocyanate
SMILES 字串
FC(F)(F)c1cc(cc(c1)C(F)(F)F)N=C=O
InChI
1S/C9H3F6NO/c10-8(11,12)5-1-6(9(13,14)15)3-7(2-5)16-4-17/h1-3H
InChI 密鑰
NRSSOFNMWSJECS-UHFFFAOYSA-N
應用
3,5-双(三氟甲基)苯基异氰酸酯可用于以下研究:
- 氨基官能化模型表面的化学衍生。
- 通过与4-吡咯烷基二吡啶的放热反应制备芳基氨基硫代羰基吡啶鎓两性离子盐。
- (S)-3-羟甲基-2-甲氧基甲氧基-2′-(3-(3,5-双(三氟甲基)苯基)尿酰基-苄基)-1,10-联萘的合成。
- 通过与2-氨基吡啶在MeCN中反应,合成1-[3,5-双(三氟甲基)苯基]-3-(2-吡啶基)硫脲。
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
154.4 °F - closed cup
閃點(°C)
68 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Huadong Yue et al.
Acta crystallographica. Section E, Structure reports online, 64(Pt 5), o858-o858 (2008-01-01)
The title compound, C(14)H(9)F(6)N(3)S, exhibits a nearly planar conformation in the solid state, with a dihedral angle between the planes of the benzene and pyridine rings of 14.86 (3)°. The pyridine N atom allows for the formation of a six-membered N-H⋯N(py)
Utsab Manna et al.
Dalton transactions (Cambridge, England : 2003), 46(35), 11956-11969 (2017-08-31)
A rationally designed ortho-phenylenediamine based trifluoromethyl meta-disubstituted bis-urea receptor (L) exhibits effective, consistent and systematic binding in its neutral form towards smaller spherical halides (fluoride, chloride, bromide and iodide), and relatively larger planar carbonate and tetrahedral sulphate oxyanions. All the
Kazuaki Ishihara et al.
Organic letters, 10(11), 2187-2190 (2008-04-30)
The exothermic reaction of 3,5-bis(trifluoromethyl)phenyl or 4-nitrophenyl isothiocyanate with 4-pyrrolidinopyridine (PPY) gave the corresponding arylaminothiocarbonylpyridinium salts in quantitative yields. These novel zwitterionic salts were effective as organocatalysts for the transesterification reaction of an equimolar mixture of methyl carboxylates and alcohols
Effects of ring substituents on enantioselective recognition of amino alcohols and acids in uryl-based binol receptors.
Nandhakumar R, et al.
Tetrahedron, 64(33), 7704-7708 (2008)
Nora Graf et al.
Analytical and bioanalytical chemistry, 396(2), 725-738 (2009-11-06)
The determination of amino groups on surfaces capable of binding biomolecules is important for the understanding and optimization of technologically relevant coupling processes. In this study, three different types of amino-functionalized model surfaces, amino thiolate on Au, amino siloxane on
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门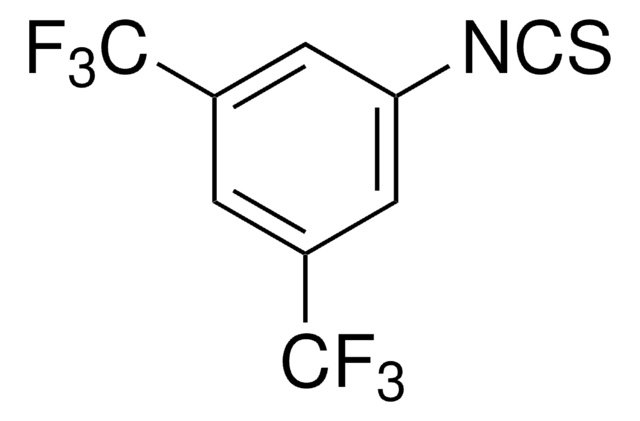


![1-[3,5-bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(-)-2-(dimethylamino)cyclohexyl]thiourea AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/236/021/d944889d-2233-4700-9f2c-caa3652d0124/640/d944889d-2233-4700-9f2c-caa3652d0124.png)
![1,3-Bis[3,5-bis(trifluoromethyl)phenyl]thiourea](/deepweb/assets/sigmaaldrich/product/structures/191/427/0218c99c-65b9-4963-938c-c47a5790dfc5/640/0218c99c-65b9-4963-938c-c47a5790dfc5.png)
![(R)-N-[(1R,2R)-2-(3-(3,5-双(三氟甲基)苯基)脲基)环己基]-叔丁基亚磺酰胺 96%](/deepweb/assets/sigmaaldrich/product/structures/389/070/18847164-c6a7-4b4e-abcb-2dbc22493a2d/640/18847164-c6a7-4b4e-abcb-2dbc22493a2d.png)