推荐产品
蒸汽壓力
<0.01 mmHg ( 20 °C)
品質等級
化驗
97%
形狀
liquid
折射率
n20/D 1.532 (lit.)
bp
257 °C (lit.)
mp
−45 °C (lit.)
密度
0.94 g/mL at 25 °C (lit.)
SMILES 字串
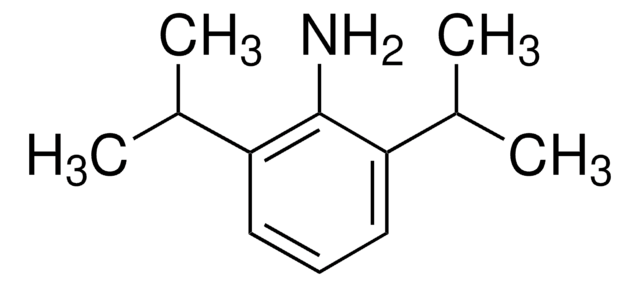
CC(C)c1cccc(C(C)C)c1N
InChI
1S/C12H19N/c1-8(2)10-6-5-7-11(9(3)4)12(10)13/h5-9H,13H2,1-4H3
InChI 密鑰
WKBALTUBRZPIPZ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2,6-二异丙基苯胺是一种胺。它在对甲苯磺酸存在的情况下与三乙酰甲烷在甲苯中发生缩合,生成3-[1-(2,6-二异丙基苯基氨基)亚乙基]戊烷-2,4-二酮。
2,6-二异丙基苯胺是一种重要的有机中间体,广泛应用于塑料和染料的合成。
2,6-二异丙基苯胺是一种重要的有机中间体,广泛应用于塑料和染料的合成。
2,6-二异丙基苯胺是一种芳香胺。它与大体积的酰氨基吡啶基(Ap)和脒基(Amd)配体负载的双(三甲基甲硅烷基甲基)钇络配合物反应,生成烷基苯胺基钇。该反应涉及TMS (三甲基硅烷)的消除。
2,6-二异丙基苯胺是一种重要的有机中间体,广泛应用于塑料和染料的合成。 .
2,6-二异丙基苯胺是一种重要的有机中间体,广泛应用于塑料和染料的合成。 .
應用
2,6-二异丙基苯胺可用于制备基于萘二酰亚胺(NDI)的有机催化剂。
2,6-二异丙基苯胺可用于制备多配位希夫碱配体前体。它可用于制备NSN-供体前配体4,5-双(2,6-二异丙基苯胺基)-2,7-二叔丁基-9,9-二甲基硫杂蒽。它可用于制备N-杂环碳烯配合物,以用于无环酮的α-芳基化、卤代芳烃的胺化和水性Suzuki偶联反应。
訊號詞
Warning
危險聲明
危險分類
Aquatic Chronic 3 - Eye Irrit. 2
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 2
個人防護裝備
Eyeshields, Gloves
其他客户在看
Reactions of Bis (alkyl) yttrium Complexes Supported by Bulky N, N Ligands with 2, 6-Diisopropylaniline and Phenylacetylene.
Karpov AV, et al.
Organometallics, 31(15), 5349-5357 (2012)
Kouki Matsubara et al.
The Journal of organic chemistry, 72(14), 5069-5076 (2007-06-15)
Arylation of both acyclic ketones and primary and secondary amines was achieved using a new, simple, stable, and easy-to-access nickel(II)-halide complex bearing mixed PPh3/N-heterocyclic carbene ligands as a catalyst precursor. Acyclic ketones were first arylated at the alpha-position with the
Christoph Fleckenstein et al.
Chemical communications (Cambridge, England), (27), 2870-2872 (2007-07-05)
Sulfonated, water-soluble imidazolium and imidazolinium salts were synthesized and the respective Pd-complexes with N,N'-bis(2,6-dialkyl-4-SO(3)(-)-phenyl)imidazol-2-ylidene and N,N'-bis(2,6-dialkyl-4-SO(3)(-)-phenyl)-4,5-dihydroimidazol-2-ylidene ligands were applied in aqueous Suzuki coupling reactions of aryl chlorides.
Electron-deficient naphthalene diimides as efficient planar Π-acid organocatalysts for selective oxidative C-C coupling of 2, 6-di-tert-butylphenol: A temperature effect.
Ke H, et al.
J. Mol. Catal. A: Chem., 385, 26-30 (2014)
Balamurugan Vidjayacoumar et al.
Dalton transactions (Cambridge, England : 2003), 41(26), 8175-8189 (2012-05-09)
A rigid NSN-donor proligand, 4,5-bis(2,6-diisopropylanilino)-2,7-di-tert-butyl-9,9-dimethylthioxanthene (H(2)[TXA(2)], 1) was prepared by palladium-catalyzed coupling of 2,6-diisopropylaniline with 4,5-dibromo-2,7-di-tert-butyl-9,9-dimethylthioxanthene. Deprotonation of 1 using (n)BuLi provided Li(2)(DME)(2)[TXA(2)] (2), and subsequent reaction with UCl(4) afforded [Li(DME)(3)][(TXA(2))UCl(3)] (4). The analogous NON-donor ligated complex [(XA(2))UCl(3)K(DME)(3)] [3; XA(2)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门