推荐产品
品質等級
化驗
98%
bp
261 °C (lit.)
mp
40-42 °C (lit.)
官能基
bromo
nitro
儲存溫度
2-8°C
SMILES 字串
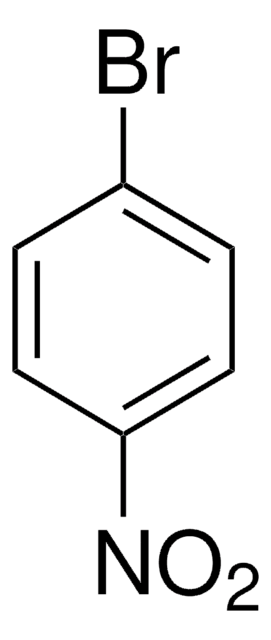
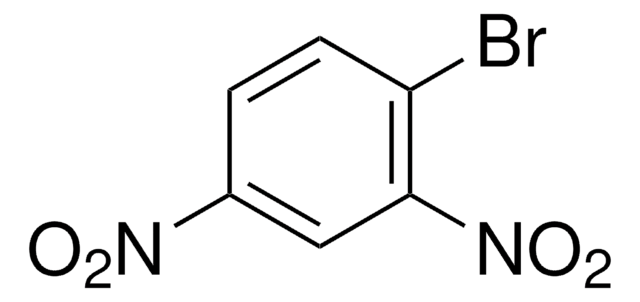
[O-][N+](=O)c1ccccc1Br
InChI
1S/C6H4BrNO2/c7-5-3-1-2-4-6(5)8(9)10/h1-4H
InChI 密鑰
ORPVVAKYSXQCJI-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
1-溴-2-硝基苯与一系列 β-卤代烯醛,-烯酮或 -酯发生钯[0]介导的 Ullmann 交叉偶联反应,得到相应的 β-芳基衍生物。研究报道了 1-溴-2-硝基苯与 β-溴-α,β-不饱和醛的钯[0]介导的 Ullmann 交叉偶联反应。
應用
1-溴-2-硝基苯可用于制备:
- 4-甲氧基-2′-硝基二苯醚
- 1-甲氧基-3,5-二-(2-硝基-苯氧基)苯
- 5-羟基-3-甲氧基-2′-硝基二苯醚
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
233.6 °F - closed cup
閃點(°C)
112 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Martin G Banwell et al.
Organic letters, 6(16), 2741-2744 (2004-07-30)
Palladium[0]-mediated Ullmann cross-coupling of 1-bromo-2-nitrobenzene (1 R = H) and its derivatives with a range of beta-halo-enals, -enones, or -esters readily affords the corresponding beta-aryl derivatives, which are converted into the corresponding quinolines, 2-quinolones, phenanthridines, or 6(5H)-phenanthridinones on reaction with
New protocols for the synthesis of 3, 4-annulated and 4-substituted quinolines from ?-bromo-a, ?-unsaturated aldehydes and 1-bromo-2-nitrobenzene or 2-bromoacetanilide.
Some S, et al.
Tetrahedron Letters, 48(20), 3609-3612 (2007)
4-Hydroxy-2'-Nitrodiphenyl Ether Analogues as Novel Tyrosinase Inhibitors.
Sapkota K, et al.
Bull. Korean Chem. Soc., 31(5), 1319-1319 (2010)
Luka A Wright et al.
Dalton transactions (Cambridge, England : 2003), 44(16), 7230-7241 (2015-03-20)
The 2-(2′-aniline)-6-imine-pyridines, 2-(C6H4-2′-NH2)-6-(CMe=NAr)C5H3N (Ar = 4-i-PrC6H4 (HL1a), 2,6-i-Pr2C6H3 (HL1b)), have been synthesised via sequential Stille cross-coupling, deprotection and condensation steps from 6-tributylstannyl-2-(2-methyl-1,3-dioxolan-2-yl)pyridine and 2-bromonitrobenzene. The palladium(II) acetate N,N,N-pincer complexes, [{2-(C6H4-2′-NH)-6-(CMe=NAr)C5H3N}Pd(OAc)] (Ar = 4-i-PrC6H4 (1a), 2,6-i-Pr2C6H3 (1b)), can be prepared by
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门