所有图片(3)
About This Item
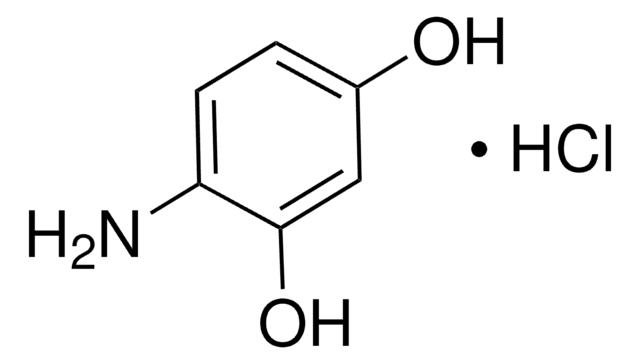
线性分子式:
(H2N)2C6H3OH
CAS号:
分子量:
124.14
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
mp
161-165 °C (lit.)
SMILES 字串
Nc1cccc(O)c1N
InChI
1S/C6H8N2O/c7-4-2-1-3-5(9)6(4)8/h1-3,9H,7-8H2
InChI 密鑰
PCAXITAPTVOLGL-UHFFFAOYSA-N
一般說明
2,3-Diaminophenol is an aromatic diamine and forms Pd(II) and Pt(II) complexes. 2,3-Diaminophenol reacts with 2,4-pentanedione to yield the corresponding benzo[b][1,4]diazepinium salts. 2,3-Diaminophenol reacts with salicylaldehyde or 5-bromosalicylaldehyde in absolute ethanol to yield new unsymmetrical Schiff base.
應用
2,3-Diaminophenol was used:
- in one-pot microwave assisted synthesis of amino-1,5-benzoxazepines and hydroxyl-1,5-benzodiazepines
- in the electrosynthesis of poly(2,3-diaminophenol) via electro-oxidation
- in a synthesis of tetradentate Schiff base complexes via reaction with salicylaldehyde or 5-bromosalicylaldehyde and metals such as Mn(III), Ni(II) and Cu(II)
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Unsymmetrical tetradentate Schiff base complexes derived from 2, 3-diaminophenol and salicylaldehyde or 5-bromosalicylaldehyde.
Ourari A, et al.
Transition Metal Chemistry, 31(2), 169-175 (2006)
Transition Met. Chem. (London), 31, 169-169 (2006)
Electropolymerization of 2, 3-diaminophenol.
Del Valle MA, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 38(9), 1698-1703 (2000)
Andreas Schmidt et al.
Organic & biomolecular chemistry, 1(23), 4342-4350 (2003-12-20)
2,3-Diaminophenol 4, 3,4-diaminophenol 5, 4-methoxy-1,2-diaminobenzene 6, 3,4-diaminobenzenethiol 7, 2,3-diaminobenzoic acid 8, and 3,4-diaminobenzoic acid 9 were reacted with 2,4-pentanedione to yield the corresponding benzo[b][1,4]diazepinium salts, respectively. The hydroxy-benzo[b][1,4]diazepinium salts 17 and 18 do not form mesomeric betaines (MB) on deprotonation.
Constantinos G Neochoritis et al.
Journal of medicinal chemistry, 53(23), 8409-8420 (2010-11-06)
Amino-1,5-benzoxazepines 2 and 5 and hydroxyl-1,5-benzodiazepines 3 and 6 have been synthesized in one-pot solvent-free conditions from 2,3-diaminophenol and ketones through microwave assisted acid catalysis, the benzoxazepine/benzodiazepine ratio depending on the R(1) and R(3) aryl substituents. The otherwise inaccessible and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门