推荐产品
化驗
97%
形狀
solid
mp
80-83 °C (lit.)
SMILES 字串
C1COCCN(CCOCCOCCN(CCO1)Cc2ccccc2)Cc3ccccc3
InChI
1S/C26H38N2O4/c1-3-7-25(8-4-1)23-27-11-15-29-19-21-31-17-13-28(24-26-9-5-2-6-10-26)14-18-32-22-20-30-16-12-27/h1-10H,11-24H2
InChI 密鑰
WAHZGOBRKWVALN-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
7,16-Dibenzyl-1,4,10,13-tetraoxa-7,16-diazacyclooctadecane can be used:
- As a macrocycle that facilitates the modification of the electrode used in the estimation of riboflavin or vitamin B2 in food and pharmaceutical samples.
- As an ionophore in the preparation of poly(vinyl chloride) membrane-based Zn2+ sensor applicable in the estimation of Zn in water and drug samples.
- To prepare a modified graphite electrode, which is used in the detection of samarium in ores and industrial effluents.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Fran Supek et al.
European journal of medicinal chemistry, 46(8), 3444-3454 (2011-06-02)
18-crown-6 ethers are known to exert their biological activity by transporting K(+) ions across cell membranes. Using non-linear Support Vector Machines regression, we searched for structural features that influence antiproliferative activity in a diverse set of 19 known oxa-, monoaza-
Marko Marjanović et al.
Journal of medicinal chemistry, 50(5), 1007-1018 (2007-02-16)
The present paper demonstrates the antiproliferative ability and structure-activity relationships (SAR) of 14 crown and aza-crown ether analogues on five tumor-cell types. The most active compounds were di-tert-butyldicyclohexano-18-crown-6 (3), which exhibited cytotoxicity in the submicromolar range, and di-tert-butyldibenzo-18-crown-6 (5) (IC50
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门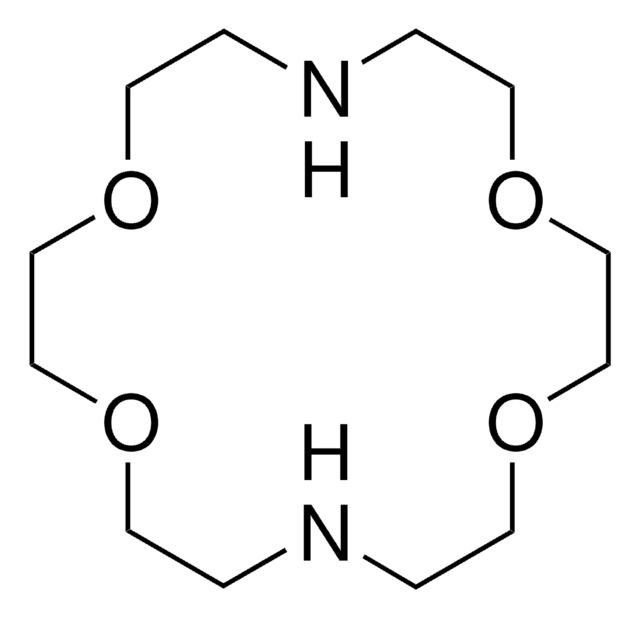
![4,7,13,16,21,24-六氧-1,10-二氮双环[8.8.8]二十六烷 98%](/deepweb/assets/sigmaaldrich/product/structures/189/812/8a6555e5-8de6-4236-865f-19339cee3634/640/8a6555e5-8de6-4236-865f-19339cee3634.png)
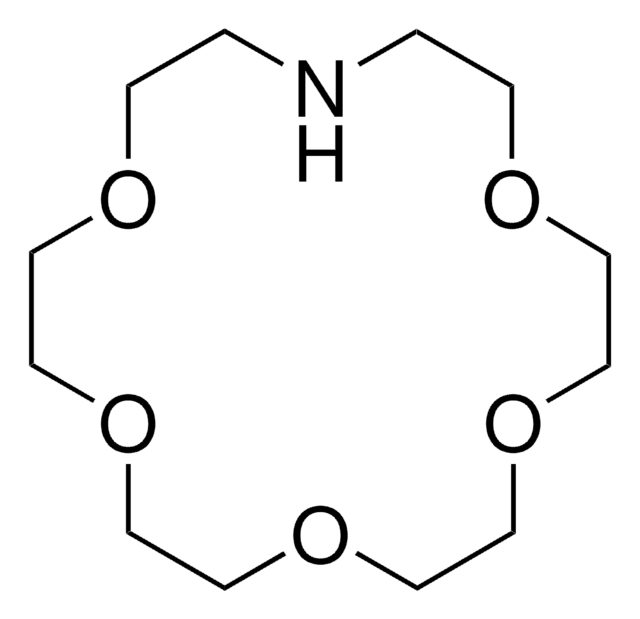
![4,7,13,16,21-五氧杂-1,10-二氮杂二环[8.8.5]廿三烷 98%](/deepweb/assets/sigmaaldrich/product/structures/444/464/eeb08f63-862e-447e-8e41-342d713f439c/640/eeb08f63-862e-447e-8e41-342d713f439c.png)