推荐产品
品質等級
化驗
≥97.0% (GC)
折射率
n20/D 1.420
bp
40-42 °C/0.1 mmHg (lit.)
密度
0.988 g/mL at 20 °C (lit.)
官能基
ester
nitrile
儲存溫度
2-8°C
SMILES 字串
CC(C)(C)OC(=O)CC#N
InChI
1S/C7H11NO2/c1-7(2,3)10-6(9)4-5-8/h4H2,1-3H3
InChI 密鑰
BFNYNEMRWHFIMR-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
tert-Butyl cyanoacetate undergoes functionalization and decarboxylation to form 3-amino-4-alkyl isoquinolines.
應用
tert-Butyl cyanoacetate was used in the synthesis of vinylogous urea.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
244.4 °F - closed cup
閃點(°C)
118 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Barry B Snider et al.
Organic letters, 7(20), 4519-4522 (2005-09-24)
[reaction: see text] Addition of the enolate of tert-butyl acetate to cyanamide methyl ester 17 followed by treatment with LHMDS afforded vinylogous urea 19 in 27% yield. Vinylogous urea 19 was also obtained from 37 and tert-butyl cyanoacetate in 50%
Ben S Pilgrim et al.
Organic letters, 15(24), 6190-6193 (2013-11-21)
A methyl ketone, an aryl bromide, an electrophile, and ammonium chloride were combined in a four-component, three-step, and one-pot coupling procedure to furnish substituted isoquinolines in overall yields of up to 80%. This protocol utilizes the palladium catalyzed α-arylation reaction
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门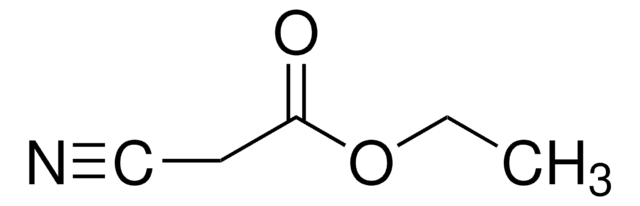




![1,8-二氮杂双环[5.4.0]十一碳-7-烯 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)