推荐产品
化驗
97%
形狀
liquid
折射率
n20/D 1.596 (lit.)
bp
141-142 °C/30 mmHg (lit.)
密度
1.091 g/mL at 25 °C (lit.)
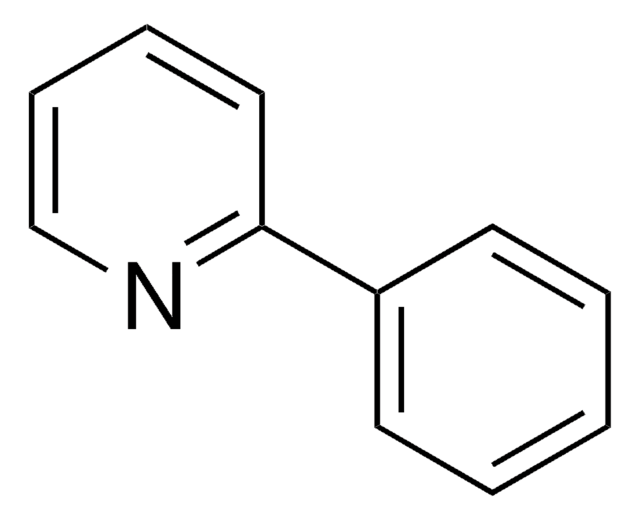
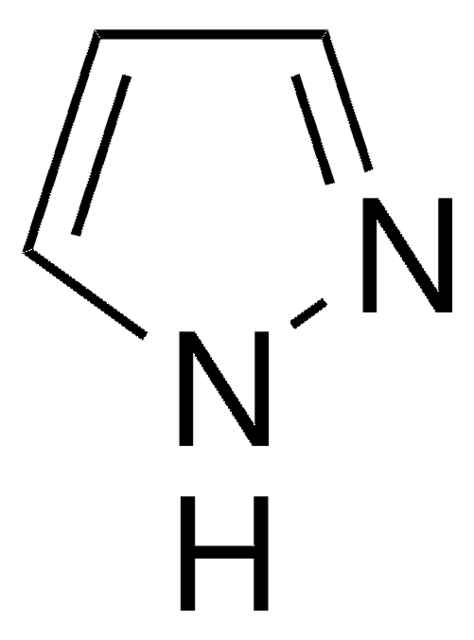
SMILES 字串
c1ccc(cc1)-n2cccn2
InChI
1S/C9H8N2/c1-2-5-9(6-3-1)11-8-4-7-10-11/h1-8H
InChI 密鑰
WITMXBRCQWOZPX-UHFFFAOYSA-N
一般說明
1-苯基吡唑可与三氯化铑进行环金属化反应,生成rac-二(μ-氯)四[2-(吡唑-1-基)苯基-C1和N2′]双铑。副产物HOAc自催化的1-苯基吡唑的C-H键活化已被研究。
應用
1-苯吡唑已被用于:
- 制备4,5-二苯基吡唑并[1,5-a]喹啉、1-(1,2,3,4-四苯基萘-5-基)吡唑和1-(1,2,3,4,5,6,7,8-八苯基蒽-9-基)吡唑
- 作为环金属配体用于制备新型杂配铱(III)配合物
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
235.4 °F - closed cup
閃點(°C)
113 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Jian-ning Yu et al.
Guang pu xue yu guang pu fen xi = Guang pu, 30(9), 2424-2427 (2010-11-26)
New heteroleptic iridium(III) complexes (ppz)2Ir(LX), which consist of two cyclometalated ligands ppz(1-phenylpyrazole) together with an ancillary ligand LX (LX= 2-(2'-hydroxylphenyl)benzothiazole (BTZ), 2-(3'-methyl-2'-hydroxylphenyl) benzothiazole (3-MeBTZ), 2-(4'-methyl-2'-hydroxylphenyl) benzothiazole (4-MeBTZ) and 2-(4'-Trifluoromethyl-2'hydroxylphenyl) benzothiazole (4-tfmBTZ)), were synthesized and characterized. The molecular structures and photophysical
Cyclometallated compounds: VII. X-Ray crystal structure of the product of cyclometallation of 1-phenylpyrazole with rhodium trichloride.
Steel PJ.
Journal of Organometallic Chemistry, 408(3), 395-402 (1991)
Indira Fabre et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(23), 7595-7604 (2013-04-19)
The activation of the C-H bond of 1-phenylpyrazole (2) and 2-phenyl-2-oxazoline (3) by [Ru(OAc)2(p-cymene)] is an autocatalytic process catalyzed by the co-product HOAc. The reactions are indeed faster in the presence of acetic acid and water but slower in the
Nobuyoshi Umeda et al.
The Journal of organic chemistry, 76(1), 13-24 (2010-12-15)
The direct oxidative coupling of phenylazoles with internal alkynes proceeds efficiently in the presence of a rhodium catalyst and a copper oxidant accompanied by double or quadruple C-H bond cleavages. Thus, as a representative example, 4,5-diphenylpyrazolo[1,5-a]quinoline, 1-(1,2,3,4-tetraphenylnaphthalen-5-yl)pyrazole, and 1-(1,2,3,4,5,6,7,8-octaphenylanthracen-9-yl)pyrazole can
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门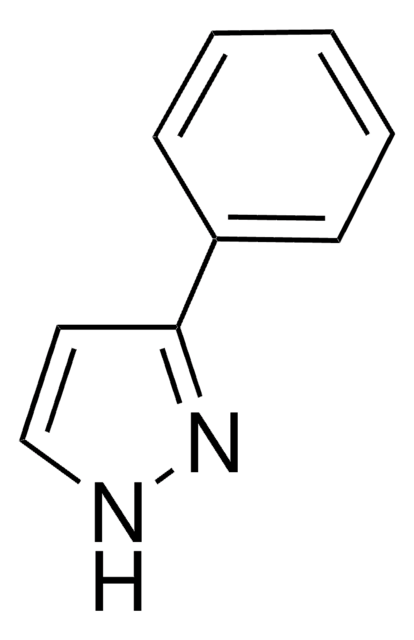
![苯并[h]喹啉 97%](/deepweb/assets/sigmaaldrich/product/structures/344/715/928932d2-4ca4-4402-b56c-85a80100ce17/640/928932d2-4ca4-4402-b56c-85a80100ce17.png)