所有图片(1)
About This Item
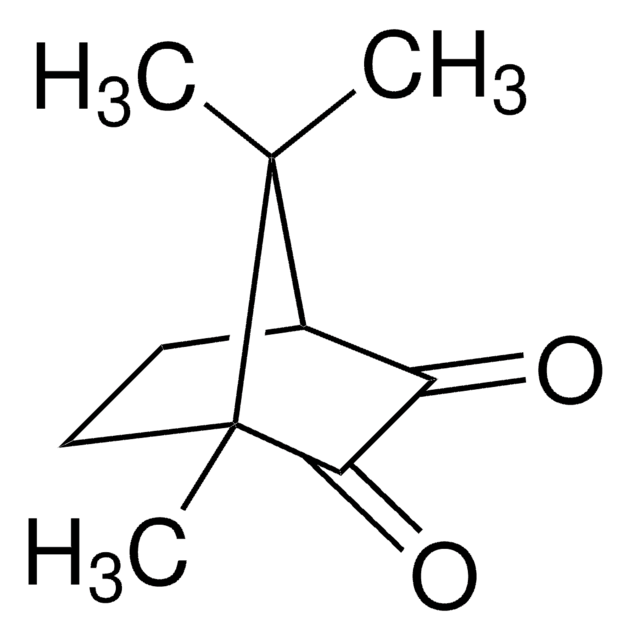
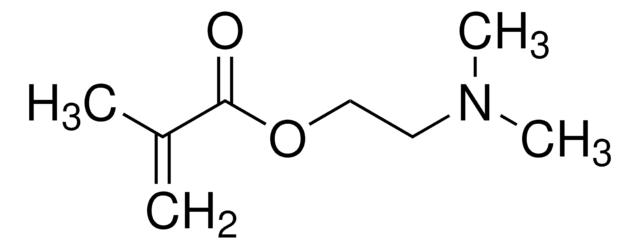
经验公式(希尔记法):
C10H14O2
CAS号:
分子量:
166.22
Beilstein:
2327696
MDL號碼:
分類程式碼代碼:
12352115
PubChem物質ID:
NACRES:
NA.22
推荐产品
品質等級
化驗
99%
光學活性
[α]20/D −101°, c = 2 in toluene
mp
200-203 °C (lit.)
官能基
ketone
SMILES 字串
CC1(C)[C@@H]2CC[C@@]1(C)C(=O)C2=O
InChI
1S/C10H14O2/c1-9(2)6-4-5-10(9,3)8(12)7(6)11/h6H,4-5H2,1-3H3/t6-,10+/m1/s1
InChI 密鑰
VNQXSTWCDUXYEZ-LDWIPMOCSA-N
正在寻找类似产品? 访问 产品对比指南
應用
(1R)-(−)-Camphorquinone can be used as a chiral starting material for the preparation of:
- α-Hydroxycamphors by selective reduction of keto groups using various vegetables.
- Camphor-1,2-diamine platinum(II) complexes for DNA interaction studies.
- Camphoric anhydride by unsensitized photo-oxidation in the presence of oxygen and polar solvents.
- Camphorquinone-based chiral homoallylic amine, which is reacted with aldehydes to produce homoallylic primary amines via imine formation followed by 2-azonia-Cope rearrangement.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Resp. Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Stereoselective reduction of ketones by various vegetables
Utsukihara T, et al.
Journal of Molecular Catalysis. B, Enzymatic, 41(3-4), 103-109 (2006)
Enantioselective transfer aminoallylation: synthesis of optically active homoallylic primary amines.
Masaharu Sugiura et al.
Journal of the American Chemical Society, 128(34), 11038-11039 (2006-08-24)
A camphorquinone-derived chiral homoallylic amine was found to react with various aldehydes via imine formation and asymmetric 2-azonia-Cope rearrangement to give optically active homoallylic primary amines. A practical level of enantioselectivity with high functional group tolerance has been attained in
Angel M Montaña et al.
Bioorganic & medicinal chemistry, 16(4), 1721-1737 (2007-11-27)
The platinum(II) complex cis-[(1S,2R,3S)-1,7,7-trimethylbicyclo[2.2.1]heptane-2,3-diamine]dichloroplatinum(II) (1) and its enantiomer (2) have been synthesized and physically and spectroscopically characterized. To obtain the enantiopure complexes the chiral pool approach was applied. The synthetic pathway has four steps, starting from (+/-)-diphenylethylenediamine (DPEDA) (3) and
Tetrahedron Asymmetry, 17, 1179-1179 (2006)
Jan Hintzpeter et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 29(1), 263-273 (2014-11-08)
The purpose of this study was to investigate the origin and function of the aldo-keto reductase (AKR) superfamily as enzymes involved in the detoxification of xenobiotics. We used the cyanobacterium Synechocystis sp. PCC 6803 as a model organism and sequence
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门