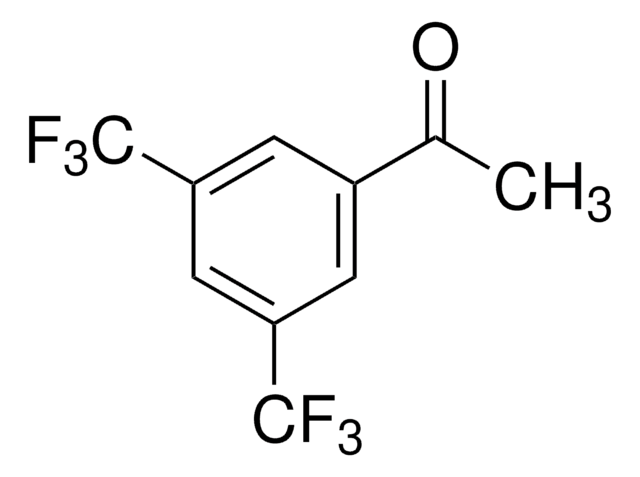
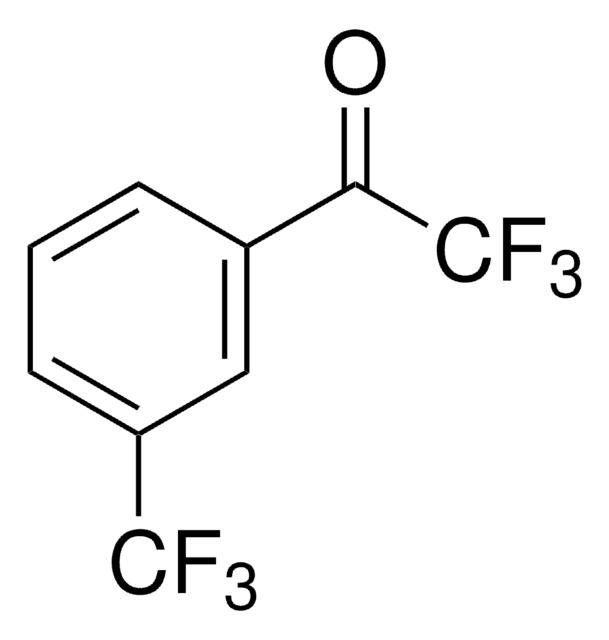
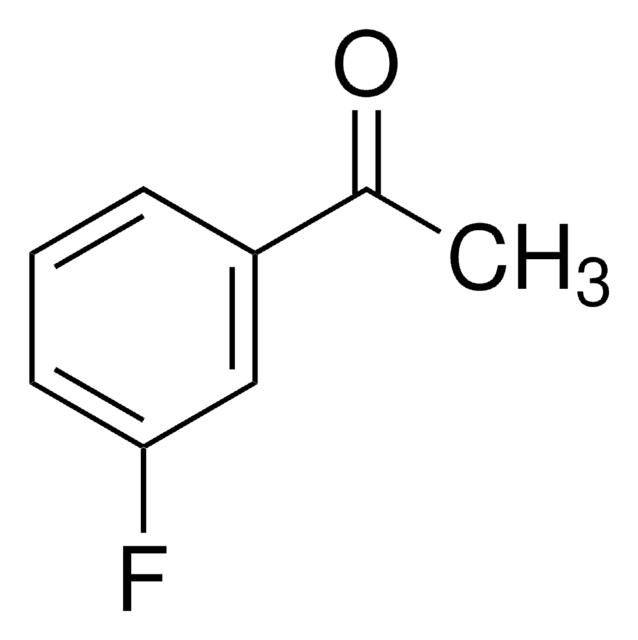
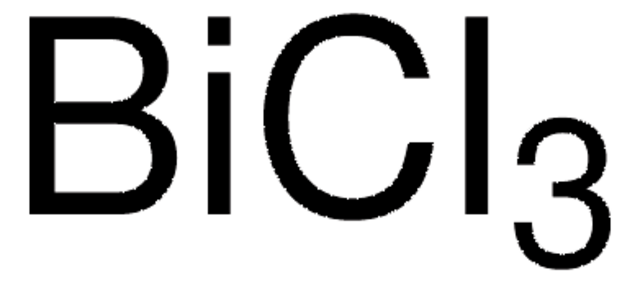推荐产品
化驗
99%
形狀
liquid
折射率
n20/D 1.4611 (lit.)
bp
198-200 °C (lit.)
密度
1.235 g/mL at 25 °C (lit.)
官能基
fluoro
ketone
SMILES 字串
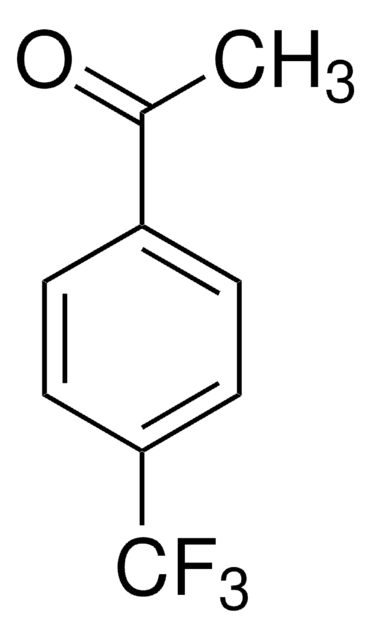
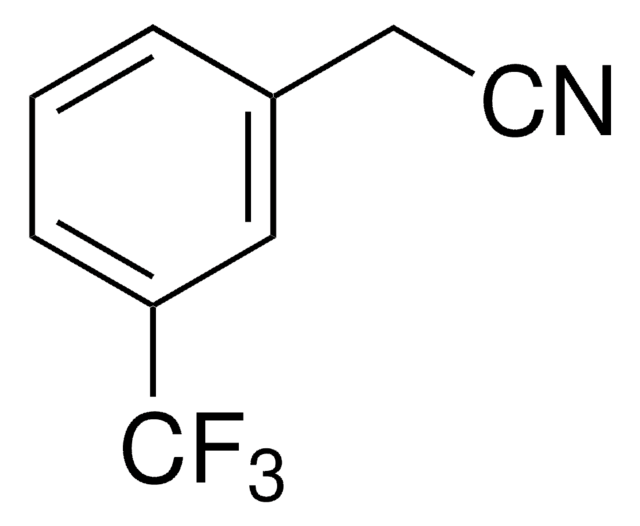
CC(=O)c1cccc(c1)C(F)(F)F
InChI
1S/C9H7F3O/c1-6(13)7-3-2-4-8(5-7)9(10,11)12/h2-5H,1H3
InChI 密鑰
ABXGMGUHGLQMAW-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Asymmetric catalytic addition of ethyl groups to 3′-(trifluoromethyl)acetophenone catalyzed by ligands derived from trans-1,2-diaminocyclohexane and camphor sulfonyl chloride has been reported. Phenylation of 3′-(trifluoromethyl)acetophenone in the presence of dihydroxy bis(sulfonamide) ligand (enantioselective catalyst), titanium tetraisopropoxide and diphenylzinc has been investigated.
應用
3′-(Trifluoromethyl)acetophenone has been used in a key step during the preparation of a commercial fungicide.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
174.2 °F - closed cup
閃點(°C)
79 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Serafino Gladiali et al.
Chemical Society reviews, 35(3), 226-236 (2006-03-01)
Hydrogen transfer reduction processes are attracting increasing interest from synthetic chemists in view of their operational simplicity and high selectivity. In this tutorial review the most significant advances recently achieved in the stereoselective reduction of unsaturated organic compounds catalyzed by
Celina García et al.
Journal of the American Chemical Society, 124(37), 10970-10971 (2002-09-13)
Many catalysts will promote the asymmetric addition of alkylzinc reagents to aldehydes. In contrast, there are no reports of additions to ketones that are both general and highly enantioselective. We describe herein a practical catalytic asymmetric addition of ethyl groups
Celina García et al.
Organic letters, 5(20), 3641-3644 (2003-09-26)
[reaction: see text] The catalytic asymmetric addition of phenyl groups from diphenylzinc to ketones is reported. The catalyst, generated from a dihydroxy bis(sulfonamide) ligand and titanium tetraisopropoxide, gives good to excellent enantioselectivities with a range of substrates.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门