About This Item
推荐产品
品質等級
化驗
98%
形狀
solid
反應適用性
core: palladium
reaction type: Cross Couplings
reagent type: catalyst
reaction type: C-H Activation
環保替代產品特色
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
環保替代類別
, Aligned
儲存溫度
2-8°C
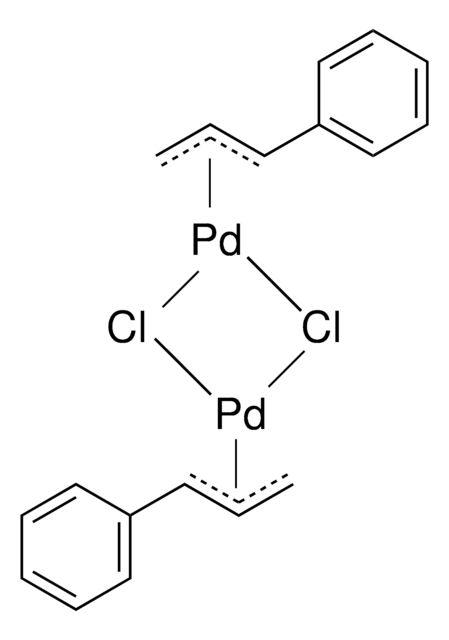
SMILES 字串
Cl[Pd]CC=C.Cl[Pd]CC=C
InChI
1S/2C3H5.2ClH.2Pd/c2*1-3-2;;;;/h2*3H,1-2H2;2*1H;;/q;;;;2*+1/p-2
InChI 密鑰
TWKVUTXHANJYGH-UHFFFAOYSA-L
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- 微波辅助Heck芳基化用阳离子钯催化剂的合成。
- N-杂环卡宾-钯-η3-烯丙基氯络合物的合成:它是芳基溴化物和活化芳基氯化物Suzuki-Miyaura交叉偶联的有效催化剂。
- 1,4-二烯丙基-1,2-二氢异喹啉的合成。
- 作为TPGS-750-M更绿色Buchwald-Hartwig耦合的催化剂
朝着通过E因子而量化的绿色过渡金属催化过程前进
訊號詞
Warning
危險聲明
危險分類
Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
商品
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
TPGS-750-M, a second generation surfactant, is useful for room temperature, palladium and ruthenium-catalyzed reactions in water. Reactions include the Heck reaction, Suzuki-Miyaura reaction, Sonogashira reaction, Buchwald-Hartwig amination reaction, Negishi reaction, and olefin metathesis.
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
实验方案
Buchwald-Hartwig Amination Reaction in Water at Room Temperature using TPGS-750-M
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| S202223-1EA | |
| 222380-100MG | |
| 222380-1G | 4061838777942 |
| 222380-10G | 4065270986597 |
| 222380-500MG | 4061838777959 |
| 222380-5G | 4061833313251 |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持![[Pd(allyl)Cl]2 Umicore](/deepweb/assets/sigmaaldrich/product/structures/367/851/7e957f32-7c31-40bf-8349-77de7cc990e4/640/7e957f32-7c31-40bf-8349-77de7cc990e4.png)



![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)


![Di-μ-mesylbis[2′-(amino-N)[1,1′-biphenyl]-2-yl-C]dipalladium(II) 95%](/deepweb/assets/sigmaaldrich/product/structures/197/956/09a1e75d-7d4a-407a-91e9-982e811baf0b/640/09a1e75d-7d4a-407a-91e9-982e811baf0b.png)