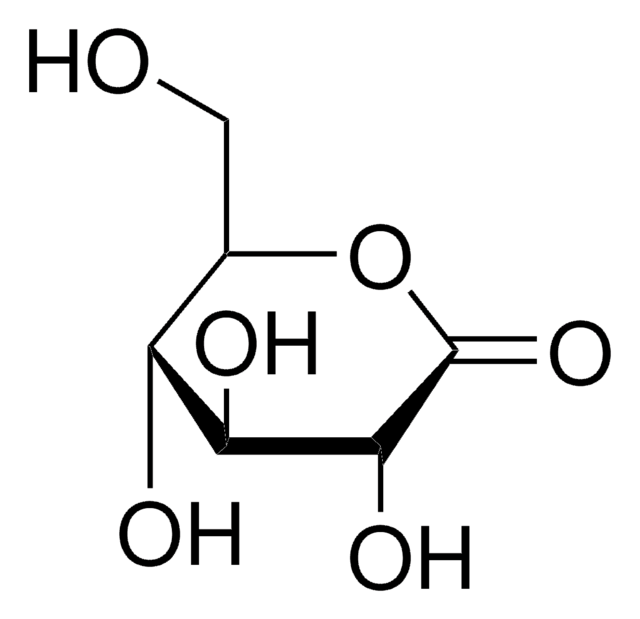
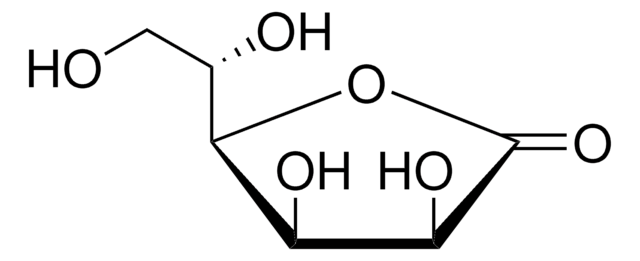
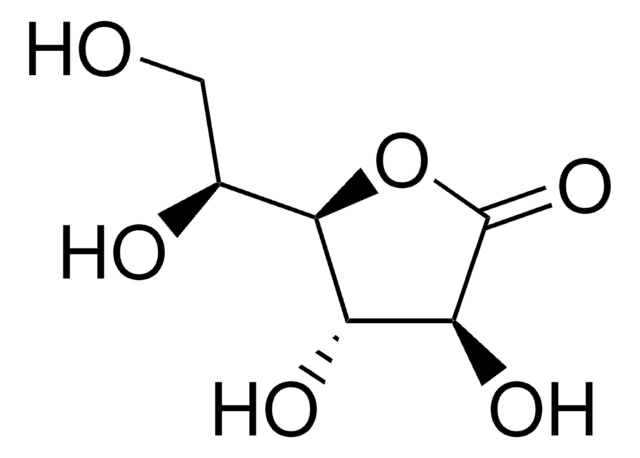
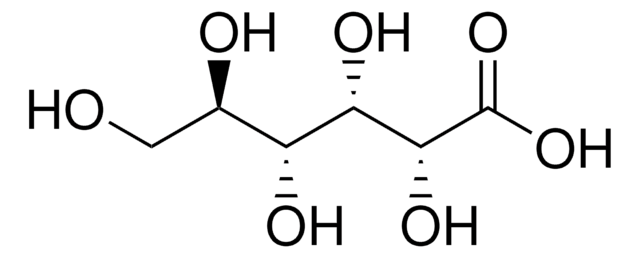
推荐产品
化驗
97%
光學活性
[α]20/D −55°, c = 4 in H2O
mp
182-188 °C (lit.)
儲存溫度
2-8°C
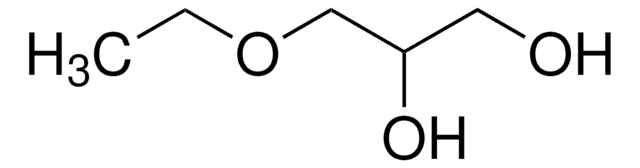
SMILES 字串
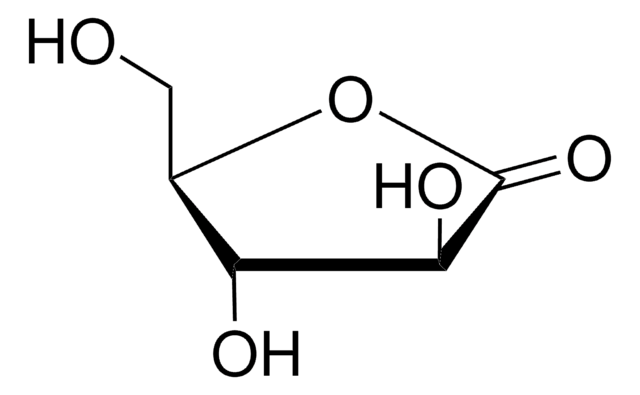
OC[C@@H](O)[C@@H]1OC(=O)[C@H](O)[C@@H]1O
InChI
1S/C6H10O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2-5,7-10H,1H2/t2-,3+,4-,5+/m1/s1
InChI 密鑰
SXZYCXMUPBBULW-LECHCGJUSA-N
正在寻找类似产品? 访问 产品对比指南
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
Ayodele O Olabisi et al.
The Journal of organic chemistry, 70(17), 6782-6789 (2005-08-13)
A convenient method to obtain unknown chiral C2- and C3-functionalized aldono-1,4-lactone derivatives starting from l-ascorbic acid, which would be valuable in the synthesis of derivatives of various pharmacologically active agents for structure-activity studies, is described. The practicality of this approach
Marjan Jeselnik et al.
Organic letters, 5(15), 2651-2653 (2003-07-19)
[reaction: see text] A new synthesis of L-noviose (11), a sugar moiety of novobiocin, is presented. D-Gulonolactone was initially converted in a few steps to the key ester derivative 7 [1-O-benzyl methyl 2,3-O-(1-methylethylidene)-alpha-L-lyxofuranosiduronate]. An appropriate selection of protecting groups enabled
G W Fleet et al.
Carbohydrate research, 205, 269-282 (1990-09-19)
The synthesis of the enantiomers of 6-epicastanospermine and of 1,6-diepicastanospermine from the enantiomeric gulonolactones is reported and the structure of the former is established as (1S,6R,7R,8R,8aR)-1,6,7,8-tetrahydroxyoctahydroindolizine. The inhibitory activities of the diastereomers against the amyloglucosidase-catalysed hydrolysis of p-nitrophenyl alpha-D-glucopyranoside were
F Puskás et al.
FEBS letters, 430(3), 293-296 (1998-08-04)
The orientation of gulonolactone oxidase activity was investigated in rat liver microsomes. Ascorbate formation upon gulonolactone addition resulted in higher intravesicular than extravesicular ascorbate concentrations in native microsomal vesicles. The intraluminal ascorbate accumulation could be prevented or the accumulated ascorbate
A Krasnov et al.
Genetic analysis : biomolecular engineering, 15(3-5), 115-119 (1999-12-22)
The reviewed studies addressed the possibility of using gene transfer for correction of L-ascorbic acid biosynthesis and carbohydrate utilization in rainbow trout. Analyses of enzymatic activities in the L-AAB pathway indicated that reasons for the lack of L-AA production can
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门