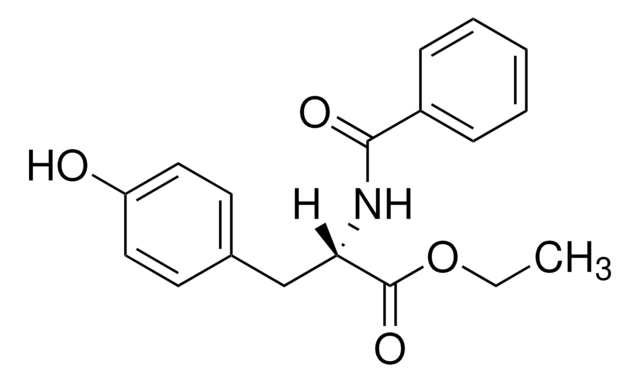
推荐产品
品質等級
化驗
99%
mp
218-221 °C (lit.)
螢光
λex 280 nm; λem 470 nm (bound to carbon anhydrase)
λem 580 in ethanol
官能基
amine
SMILES 字串
CN(C)c1cccc2c(cccc12)S(N)(=O)=O
InChI
1S/C12H14N2O2S/c1-14(2)11-7-3-6-10-9(11)5-4-8-12(10)17(13,15)16/h3-8H,1-2H3,(H2,13,15,16)
InChI 密鑰
TYNBFJJKZPTRKS-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
5-(二甲基氨基)-1-萘磺酰胺(DNSA)作为起始试剂已被用于合成2,6-二取代的吡啶、6-取代的2,2′-联吡啶和6,6′-二取代的2,2-联吡啶。它还作为荧光探针用于测定溶液中人碳酸酐酶II-DNSA的浓度。
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
A Jain et al.
Journal of medicinal chemistry, 37(13), 2100-2105 (1994-06-24)
This paper describes inhibitors for human carbonic anhydrase II (HCAII, EC 4.2.1.1) that bind with nanomolar dissociation constants. These inhibitors were developed by exploiting interactions with hydrophobic "patches" in the lip of the active site of this enzyme. These patches
Abir L Banerjee et al.
Biochemistry, 44(9), 3211-3224 (2005-03-02)
Benzenesulfonamide and iminodiacetate (IDA)-conjugated Cu(2+) independently interact at the active site and a peripheral site of carbonic anhydrases, respectively [Banerjee, A. L., Swanson, M., Roy, B. C., Jia, X., Haldar, M. K., Mallik, S., and Srivastava, D. K. (2004) J.
Jiangxiao Sun et al.
Analytical chemistry, 79(2), 416-425 (2007-01-16)
The interaction between the bovine pancrease trypsin (Tryp) and its competitive inhibitor benzamidine (1), in solution and the gas phase, is investigated using nanoflow electrospray ionization (nanoES) and Fourier transform ion cyclotron resonance mass spectrometry. In a recent study (Clark
Julia Guy et al.
Journal of the American Chemical Society, 129(39), 11969-11977 (2007-09-14)
Dimaleimide fluorogens are being developed for application to fluorescent protein labeling. In this method, fluorophores bearing two maleimide quenching groups do not fluoresce until both maleimide groups have undergone thiol addition reactions with the Cys residues of the target protein
Yongqian Xu et al.
Chemical communications (Cambridge, England), 48(92), 11313-11315 (2012-10-20)
A novel squaraine dye (SQ) exhibits improved fluorescence response toward protein detection by incorporation of a zwitterionic structure. With the aid of a dansylamide (DNSA) substituent, the new probe (DNSA-SQ) exhibits remarkable selectivity in binding to site I (a specific
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持