推荐产品
品質等級
化驗
97%
形狀
solid
mp
270-273 °C (lit.)
SMILES 字串
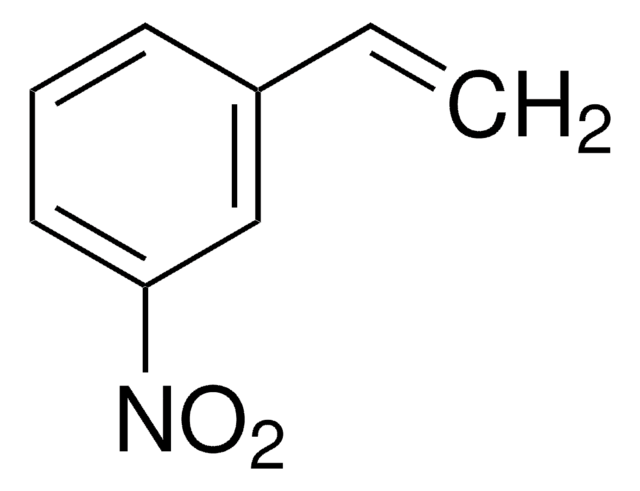
Oc1ccc2c(c1)[nH]c3ccccc23
InChI
1S/C12H9NO/c14-8-5-6-10-9-3-1-2-4-11(9)13-12(10)7-8/h1-7,13-14H
InChI 密鑰
GWPGDZPXOZATKL-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2-Hydroxycarbazole is a compound structurally related to the Ca2+-mobilizing marine toxin, 9-methyl-7-bromoeudistomin. Room temperature electronic absorption and fluorescence spectra of 2-hydroxycarbazole has been studied in concentrated aqueous potassium hydroxide solutions. It undergoes chemoselective N-alkylation using NaH as a base in a THF-DMF solvent system.
應用
2-Hydroxycarbazole was used in the synthesis of isochromene fused carbazol, (4aS,13bR)-2,5,5-trimethyl-3,4,4a,5,8,13b-hexahydroisochromeno[3,4-b]carbazole.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Tamanna Mallick et al.
Colloids and surfaces. B, Biointerfaces, 172, 440-450 (2018-09-10)
Six structurally different carbazoles (1-6) were explored as the green reducing agents for the synthesis of the fluorescent Au nanoparticles with tailor-made morphology in anionic (sodium dodecyl sulphate, SDS), cationic (cetyltrimethylammonium bromide, CTAB) and neutral (polyvinylpyrrolidone, PVP) micelle medium. Structure
Nguyen Manh Cuong et al.
Natural product communications, 4(7), 921-924 (2009-09-08)
The first synthesis of isochromene fused carbazols, (4aS, 13bR)-2,5,5-trimethyl-3,4,4a,5,8,13b-hexahydroisochromeno[3,4-b]carbazole (2) and its epi-isomer 3 by condensation of citral and 2-hydroxycarbazole using Ti(OEt)4 and MeAlC12 as catalysts is described.
S C Tovey et al.
European journal of pharmacology, 354(2-3), 245-251 (1998-10-01)
2-Hydroxycarbazole was shown to induce Ca2+ release from skeletal muscle and cardiac muscle sarcoplasmic reticulum at concentrations between 100-500 microM. This release was blocked by both 1 mM tetracaine and 30 microM ruthenium red which inhibit the ryanodine receptor or
J Doutheil et al.
Cell calcium, 25(6), 419-428 (1999-12-01)
In the physiological state, protein synthesis is controlled by calcium homeostasis in the endoplasmic reticulum (ER). Recently, evidence has been presented that dividing cells can adapt to an irreversible inhibition of the ER calcium pump (SERCA), although the mechanisms underlying
Chemoselective N-alkylation of 2-hydroxycarbazole as a model for the synthesis of N-substituted pyrrole derivatives containing acidic functions.
Albanese D, et al.
Tetrahedron, 51(19), 5681-5688 (1995)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门