推荐产品
品質等級
化驗
97%
形狀
liquid
折射率
n20/D 1.509 (lit.)
bp
206.5 °C (lit.)
mp
−11.7 °C (lit.)
密度
1.467 g/mL at 25 °C (lit.)
官能基
fluoro
nitro
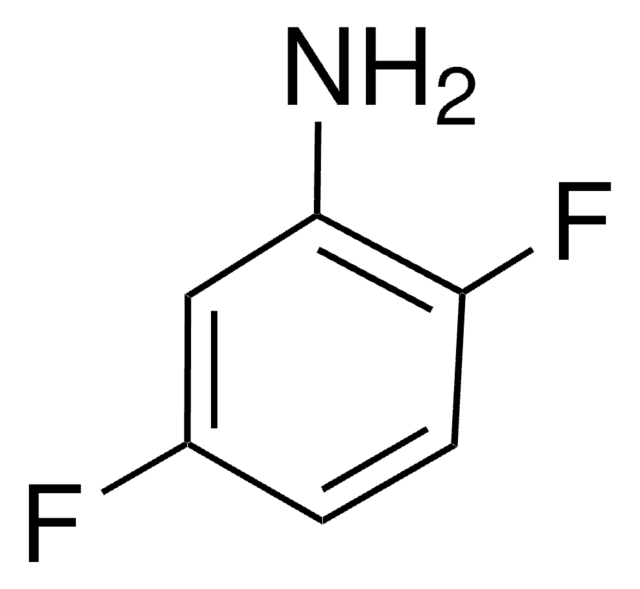
SMILES 字串
[O-][N+](=O)c1cc(F)ccc1F
InChI
1S/C6H3F2NO2/c7-4-1-2-5(8)6(3-4)9(10)11/h1-3H
InChI 密鑰
XNJAYQHWXYJBBD-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
2,5-Difluoronitrobenzene was used in the synthesis of :
- N-alkylated 2-arylaminobenzimidazoles
- quinoxalinones
- N-(2-nitro-4-fluorophenyl)-l,2,3,4-tetrahydroisoquinoline
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
194.0 °F - closed cup
閃點(°C)
90 °C - closed cup
個人防護裝備
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
H E Haenen et al.
Chemico-biological interactions, 98(2), 97-112 (1995-11-17)
Proximal tubular biotransformation of the glutathionyl (GSH) conjugate derived from 2,5-difluoronitrobenzene (5-fluoro-2-glutathionyl-nitrobenzene) was studied by means of 19F-NMR. This method allows a direct and specific detection of the fluorinated metabolites formed, at a detection limit of 1 microM for an
A solid phase traceless synthesis of quinoxalinones.
Krchnak V, et al.
Tetrahedron Letters, 41(16), 2835-2838 (2000)
Ring-opening reactions of N-aryl-, 1, 2, 3, 4-tetrahydroisoquinoline derivatives.
Andrew HK and Stanforth SP.
Tetrahedron, 48(4), 743-750 (1992)
I M Rietjens et al.
Chemico-biological interactions, 94(1), 49-72 (1995-01-01)
The in vivo metabolite patterns of 2,5-difluoroaminobenzene and of its nitrobenzene analogue, 2,5-difluoronitrobenzene, were determined using 19F NMR analysis of urine samples. Results obtained demonstrate significant differences between the biotransformation patterns of these two analogues. For the aminobenzene, cytochrome P450
Manuel A V Ribeiro da Silva et al.
The journal of physical chemistry. B, 114(40), 12914-12925 (2010-09-24)
This work reports the experimental and computational thermochemical study performed on three difluorinated nitrobenzene isomers: 2,4-difluoronitrobenzene (2,4-DFNB), 2,5-difluoronitrobenzene (2,5-DFNB), and 3,4-difluoronitrobenzene (3,4-DFNB). The standard (p° = 0.1 MPa) molar enthalpies of formation in the liquid phase of these compounds were
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门