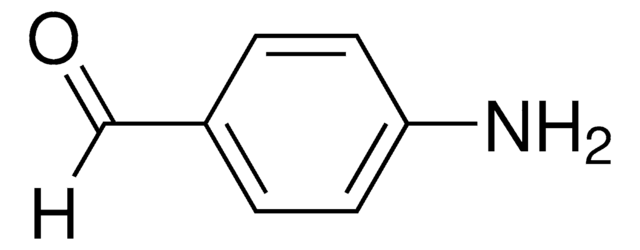
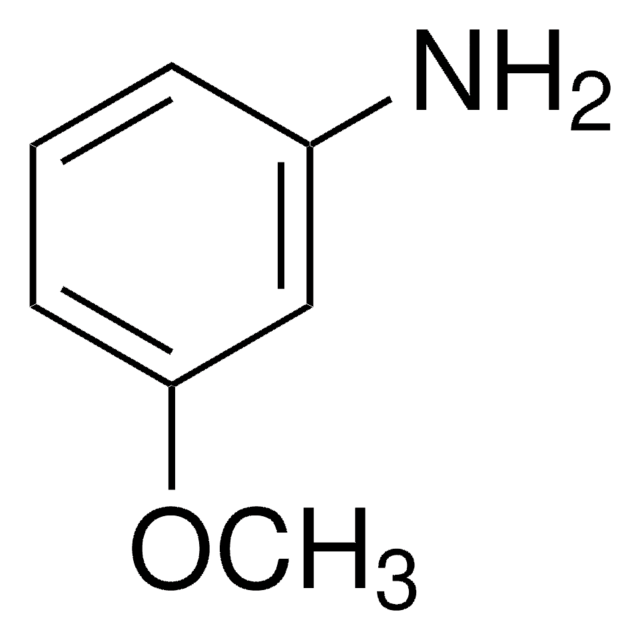
推荐产品
品質等級
化驗
99%
形狀
solid
mp
48-53 °C (lit.)
官能基
nitrile
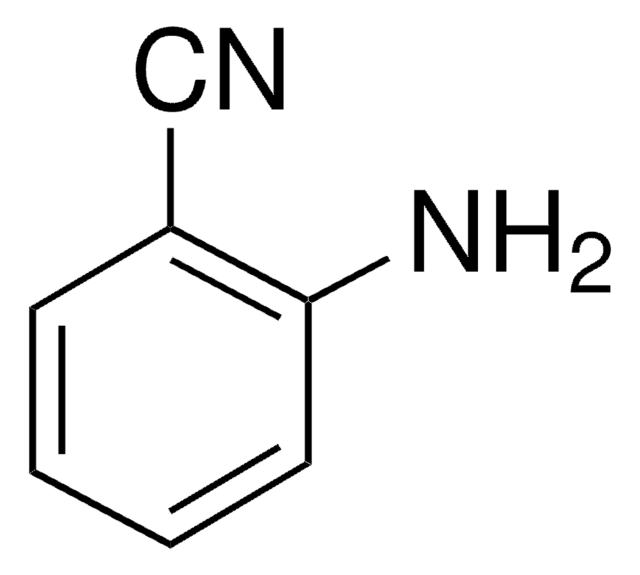
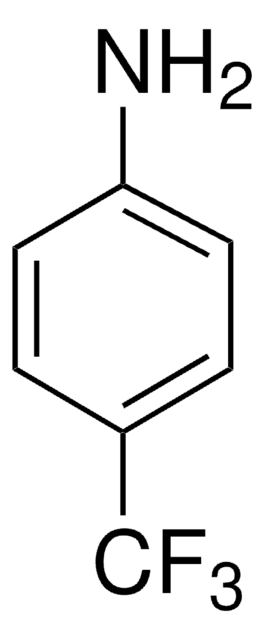
SMILES 字串
Nc1cccc(c1)C#N
InChI
1S/C7H6N2/c8-5-6-2-1-3-7(9)4-6/h1-4H,9H2
InChI 密鑰
NJXPYZHXZZCTNI-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
3-Aminobenzonitrile on condensation reaction with 4-isothiocyanato-4-methyl pentane-2-one gives condensed monocyclic pyrimidine derivatives.
應用
3-Aminobenzonitrile was used in the synthesis of series of 1-substituted-3(5)-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)pyrazoles. It was also used in the preparation of highly substituted γ-lactam analogues of a thiazolidinone follicle stimulating hormone receptor agonist.
訊號詞
Warning
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
233.6 °F - closed cup
閃點(°C)
112 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
Cheng Hua Jin et al.
European journal of medicinal chemistry, 46(9), 3917-3925 (2011-06-24)
A series of 1-substituted-3(5)-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)pyrazoles 14a-d, 15a-d, 17a, 17b, 18a-d, 19a, and 19b has been synthesized and evaluated for their ALK5 inhibitory activity in an enzyme assay and in a cell-based luciferase reporter assay. The 2-[3-(6-methylpyridin-2-yl)-4-(quinoxalin-6-yl)-1H-pyrazol-1-yl]-N-phenylethanethioamide (18a) inhibited ALK5 phosphorylation with
Sham M Sondhi et al.
Bioorganic & medicinal chemistry, 13(22), 6158-6166 (2005-08-24)
3-Aminobenzonitrile and 2-amino-4-phenyl thiazole on condensation with 4-isothiocyanato-4-methyl pentane-2-one gave condensed monocyclic pyrimidine derivatives 1 and 2, 3, respectively. Condensation of 3-aminopropyl imidazole with 3-isothiocyantobutanal gave condensed monocyclic pyrimidine derivative 4. Bicyclic pyrimidine derivatives 5a and 5b have been synthesized
Jeffrey C Pelletier et al.
Bioorganic & medicinal chemistry, 13(21), 5986-5995 (2005-08-16)
An unusual combination of Weinreb amidation and Mitsunobu lactam formation was used to prepare highly substituted gamma-lactam analogues of a thiazolidinone follicle stimulating hormone receptor agonist. The analogue synthesis was stereoselective and the final products were chemically stable. Biological properties
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门