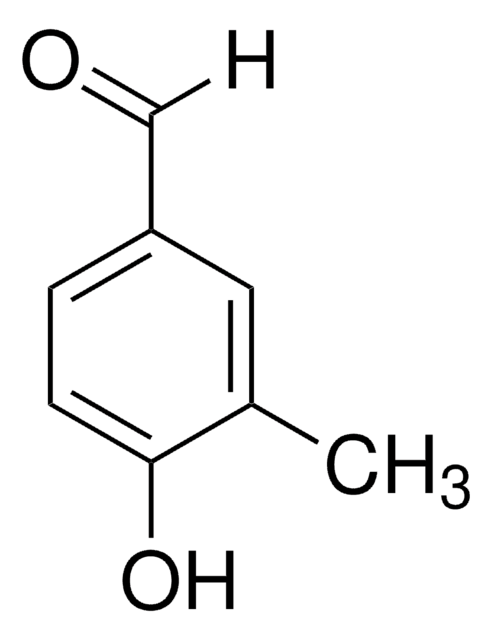
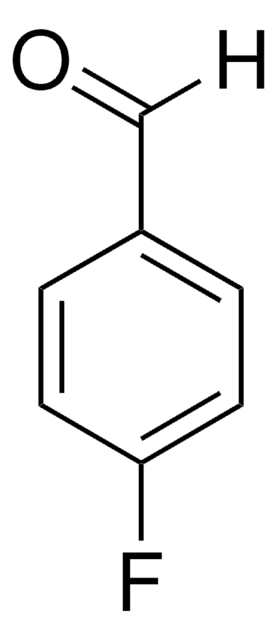
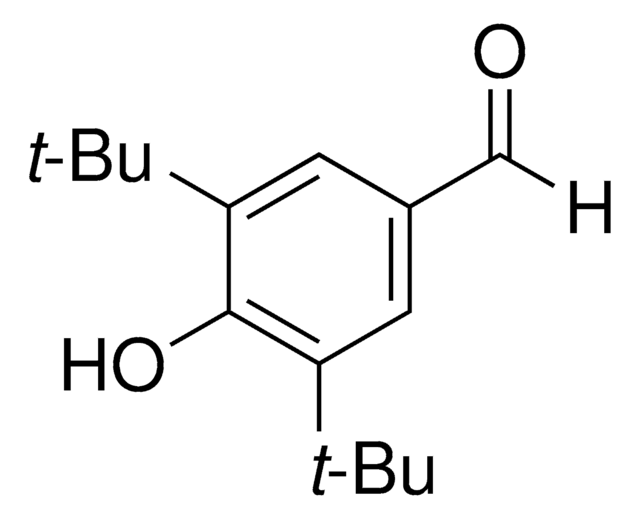
推荐产品
品質等級
化驗
95%
mp
112-114 °C (lit.)
官能基
aldehyde
SMILES 字串
[H]C(=O)c1cc(C)c(O)c(C)c1
InChI
1S/C9H10O2/c1-6-3-8(5-10)4-7(2)9(6)11/h3-5,11H,1-2H3
InChI 密鑰
UYGBSRJODQHNLQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
4-Hydroxy-3,5-dimethylbenzaldehydewas used as reagent during the phase transfer catalyzed polymerization of 4-hydroxy-3,5-dimethylbenzyl alcohol. It was used as starting reagent during the synthesis of 2,4,6-trimethylphenol.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Phase transfer catalyzed polymerization of 4-hydroxy-3, 5-dimethylbenzyl alcohol and copolymerization of 4-bromo-2, 6-dimethylphenol with 4-hydroxy-3, 5-dimethylbenzyl alcohol.
Wang JH and Percec V.
Polym. Bull., 25(1), 25-32 (1991)
A facile synthesis of 4-alkoxymethylphenols by a copper (II)-acetoxime catalyst/O2 system.
Shimizu M, et al.
Tetrahedron Letters, 32(18), 2053-2056 (1991)
P R Ortiz de Montellano et al.
The Journal of biological chemistry, 262(24), 11641-11646 (1987-08-25)
Chloroperoxidase and H2O2 oxidize styrene to styrene oxide and phenylacetaldehyde but not benzaldehyde. The epoxide oxygen is shown by studies with H2(18)O2 to derive quantitatively from the peroxide. The epoxidation of trans-[1-2H]styrene by chloroperoxidase proceeds without detectable loss of stereochemistry
D J Abraham et al.
Biochemistry, 34(46), 15006-15020 (1995-11-21)
Monoaldehyde allosteric effectors of hemoglobin were designed, using molecular modeling software (GRID), to form a Schiff base adduct with the Val 1 alpha N-terminal nitrogens and interact via a salt bridge with Arg 141 alpha of the opposite subunit. The
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门