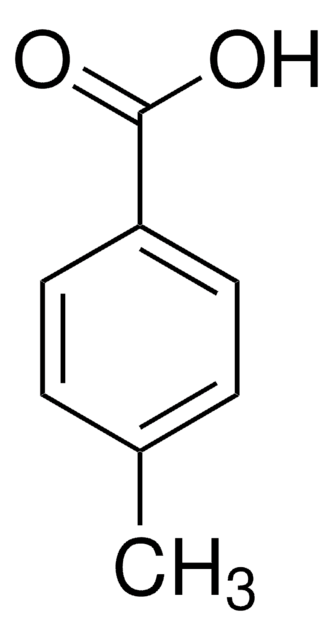
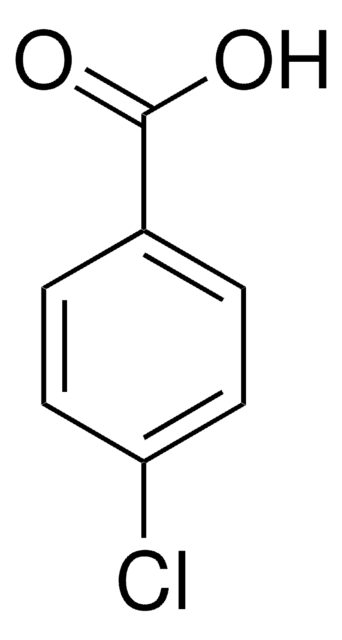
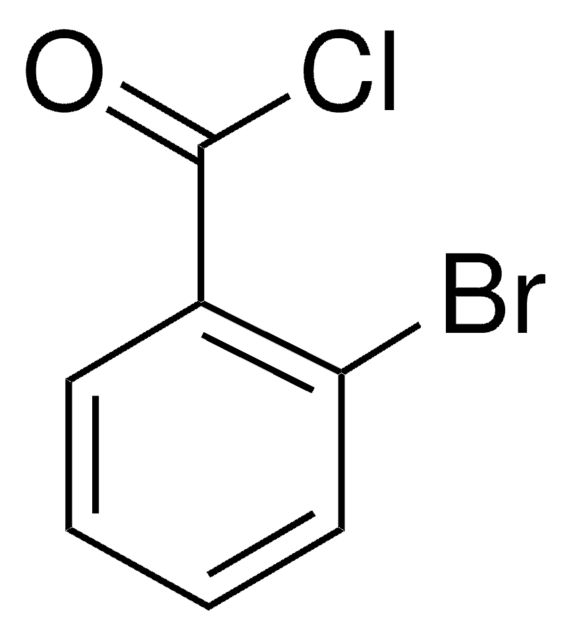
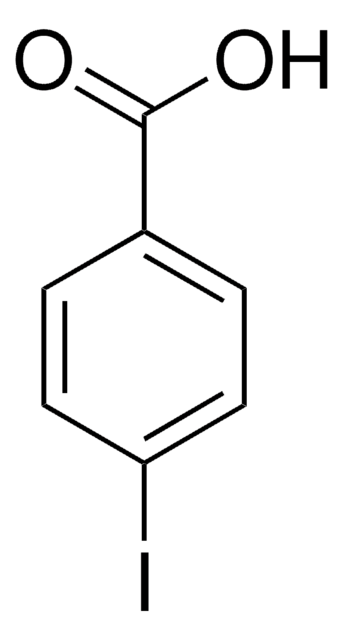
推荐产品
蒸汽壓力
>1 mmHg ( 20 °C)
化驗
97%
形狀
powder
mp
147-150 °C (lit.)
溶解度
95% ethanol: soluble 100 mg/mL, clear, colorless to yellow
SMILES 字串
OC(=O)c1ccccc1Br
InChI
1S/C7H5BrO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,(H,9,10)
InChI 密鑰
XRXMNWGCKISMOH-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
2-溴苯甲酸(2-BA)与氨基喹啉缩合,产生苯基喹啉胺。2-BA是合成各种氮杂环的常用结构单元。
應用
2-溴苯甲酸被用于合成新型锌(II)-2-溴苯甲酸盐络合物。它被用作合成半不稳定的苯并咪唑基膦配体的起始试剂。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
>212.0 °F
閃點(°C)
> 100 °C
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Thermal decomposition and antimicrobial activity of zinc (II) 2-bromobenzoates with organic ligands.
Krajnikova A, et al.
Journal of Thermal Analysis and Calorimetry, 105(2), 451-460 (2011)
Free radical reactions for heterocycle synthesis. Part 6: 2-Bromobenzoic acids as building blocks in the construction of nitrogen heterocycles.
Zhang W and Pugh G.
Tetrahedron, 59(17), 3009-3018 (2003)
J B Bongui et al.
Chemical & pharmaceutical bulletin, 49(9), 1077-1080 (2001-09-18)
Condensation of either 2-bromobenzoic acid (4) or 2-chloro-3-nitrobenzoic acid (5) with suitable aminoquinolines 6-8 afforded phenylquinolylamines 9-13. Acid mediated cyclization gave the corresponding 12H-benzo[b][1,7]phenanthrolin-7-ones 14 and 15, and 12H-benzo[b][1,10]phenanthrolin-7-ones 16-18. Compounds 14, 16, and 17 were subsequently N-methylated to 6-demethoxyacronycine
Kin Ho Chung et al.
Chemical communications (Cambridge, England), 48(14), 1967-1969 (2012-01-12)
A new class of easily accessible hemilabile benzimidazolyl phosphine ligands has been developed. The ligand skeleton is prepared from commercially available and inexpensive o-phenylenediamine and 2-bromobenzoic acid. With catalyst loading down to 0.5 mol% palladium, excellent catalytic activity towards the
K H Engesser et al.
FEMS microbiology letters, 51(1), 143-147 (1989-07-15)
Pseudomonas putida strain CLB 250 (DSM 5232) utilized 2-bromo-, 2-chloro- and 2-fluorobenzoate as sole source of carbon and energy. Degradation is suggested to be initiated by a dioxygenase liberating halide in the first catabolic step. After decarboxylation and rearomatization catechol
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门