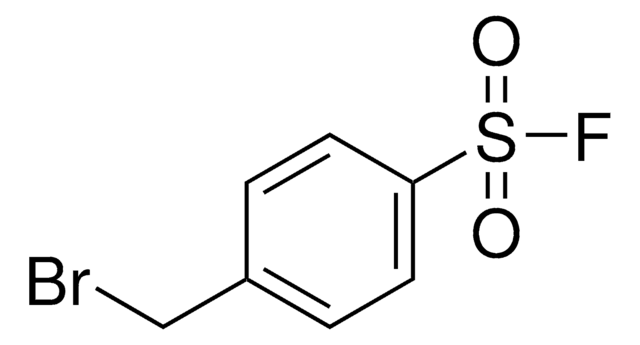
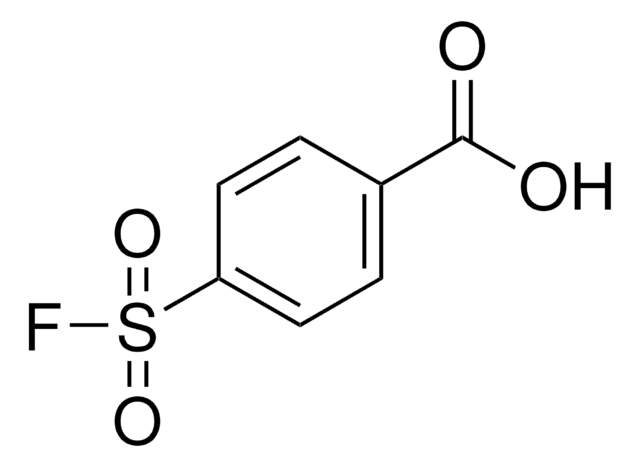
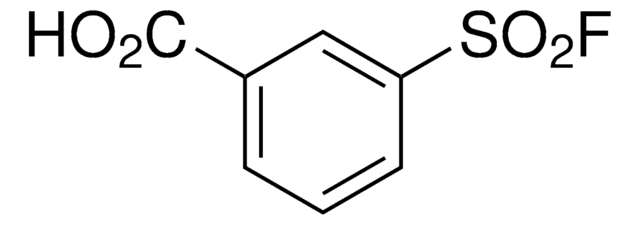
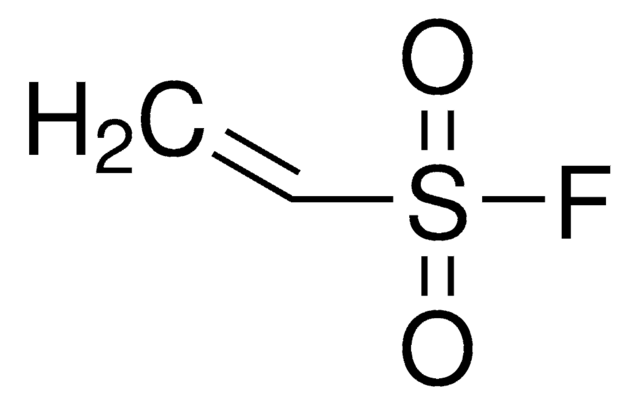
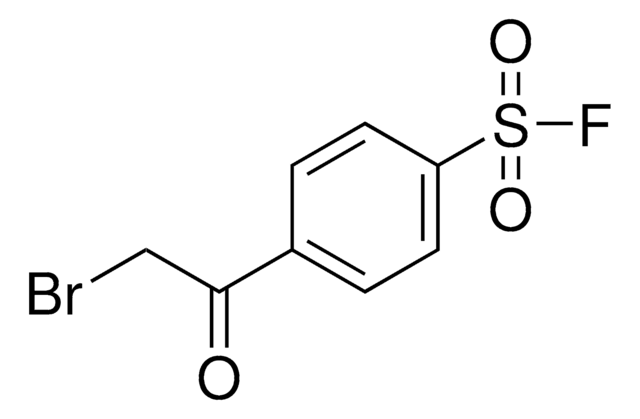
推荐产品
等級
technical grade
化驗
90%
反應適用性
reaction type: click chemistry
bp
96-97 °C/0.7 mmHg (lit.)
mp
46-48 °C (lit.)
SMILES 字串
FS(=O)(=O)c1ccc(cc1)C(Cl)=O
InChI
1S/C7H4ClFO3S/c8-7(10)5-1-3-6(4-2-5)13(9,11)12/h1-4H
InChI 密鑰
JMTAYFNTRRLWQG-UHFFFAOYSA-N
應用
4-(Fluorosulfonyl)benzoyl chloride was used as reagent in the synthesis of irreversible adenosine A1 antagonist 8-cyclopentyl-3-N-[3-((3-(4-fluorosulphonyl)benzoyl)-oxy)-propyl]-1-N-propyl-xanthine.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
J E van Muijlwijk-Koezen et al.
Bioorganic & medicinal chemistry letters, 11(6), 815-818 (2001-03-30)
A new preparative synthetic route for the irreversible adenosine A1 antagonist 8-cyclopentyl-3-N-[3-((3-(4-fluorosulphonyl)benzoyl)-oxy)-propyl]-1-N-propyl-xanthine (FSCPX, 1) is described. The availability of ample amounts of the irreversible antagonist FSCPX allowed us to use FSCPX as a research tool for adenosine A1 receptors in
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门