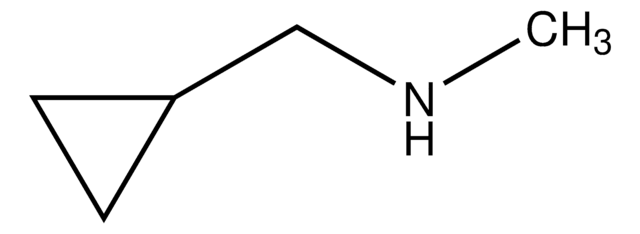
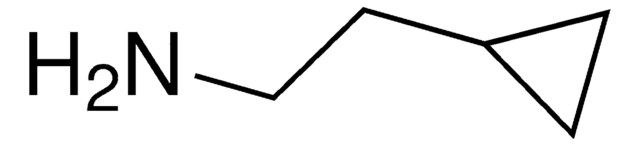
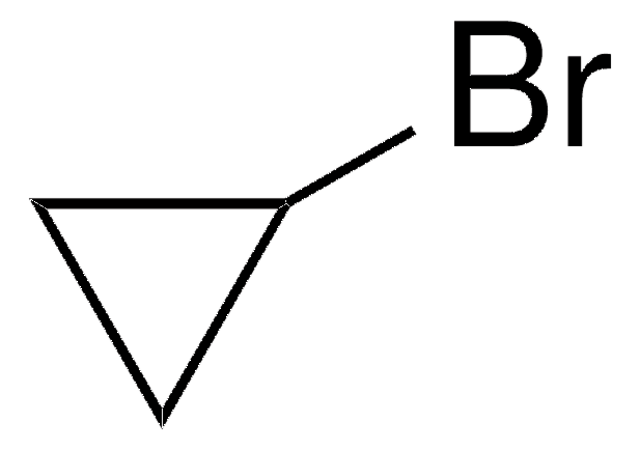
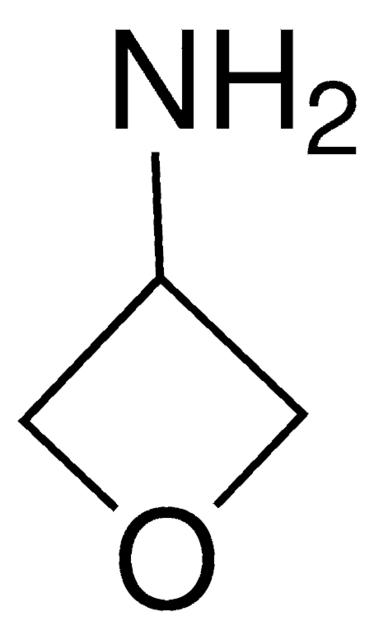
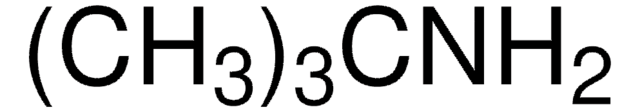推荐产品
蒸汽壓力
4.67 psi ( 20 °C)
品質等級
化驗
98%
形狀
liquid
自燃溫度
527 °F
折射率
n20/D 1.420 (lit.)
bp
49-50 °C (lit.)
密度
0.824 g/mL at 25 °C (lit.)
SMILES 字串
NC1CC1
InChI
1S/C3H7N/c4-3-1-2-3/h3H,1-2,4H2
InChI 密鑰
HTJDQJBWANPRPF-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
环丙胺 (CPA) 用于 N -\ [4-(4-氟)苯基-2-氨基噻唑-5-基] 嘧啶-2-基-烷基胺衍生物的合成 。已用于合成 Pt (CPA) 2 ( 双甲硫亚甲基丙二酸酯)和 Pt (CPA) 2 (双 乙基硫亚甲基丙二酸酯)配合物 。
生化/生理作用
环丙胺通过一种机制使细胞色素 P450 酶失活,该机制包括在氮气中先发生单电子氧化,然后环丙烷环断裂,导致酶的共价修饰 。它是 脱氮副球菌 中一种基于机制的喹啉甲胺脱氢酶抑制剂。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
33.8 °F - closed cup
閃點(°C)
1 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles
Synthesis and Antifungal Activity of 5-[2-(Alkylamino) pyrimidin-4-yl]-4-phenylthiazol-2-cycloalkylamine Derivatives on Phytophthora capsici.
Nam Sw, et al.
J. Korean Chem. Soc., 54(3), 395-402 (2011)
Coordination Mode vs. Anticancer Activity of the Platinum (II) Complexes Involving Sulfur-Containing Ylidenemalonate Ligands.
Sakai N, et al.
Bull. Korean Chem. Soc., 19(12), 1377-1379 (1998)
Dapeng Sun et al.
FEBS letters, 517(1-3), 172-174 (2002-06-14)
Cyclopropylamine is a mechanism-based inhibitor of the quinoprotein methylamine dehydrogenase (MADH) from Paracoccus denitrificans. The resulting inactivation is accompanied by the formation of a covalent cross-link between the alpha and beta subunits of MADH. The results of site-directed mutagenesis studies
Bram Denolf et al.
Organic letters, 9(2), 187-190 (2007-01-16)
Treatment of novel chiral N-sulfinyl alpha-chloro ketimines with Grignard reagents resulted in the synthesis of chiral N-(1-substituted cyclopropyl)-tert-butanesulfinamides in acceptable to good yields and diastereoselectivity via 1,3-dehydrohalogenation and subsequent addition of the Grignard reagent to the intermediate cyclopropylideneamine. Only in
Soda Chanthamath et al.
Organic letters, 15(4), 772-775 (2013-01-31)
The Ru(II)-Pheox-catalyzed asymmetric cyclopropanation of vinylcarbamates with diazoesters resulted in the corresponding cyclopropylamine derivatives in high yield and excellent diastereoselectivity (up to 96:4) and enantioselectivity (up to 99% ee).
Global Trade Item Number
| 货号 | GTIN |
|---|---|
| 125504-25G | 4061838723628 |
| 125504-10G | 4061838723611 |
| 125504-10KG |
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持